

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM19765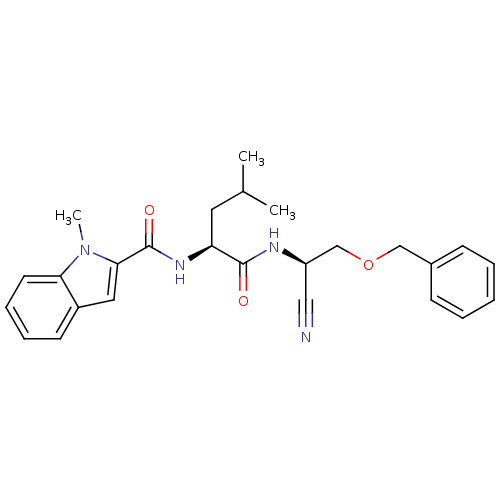 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19764 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-(1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19762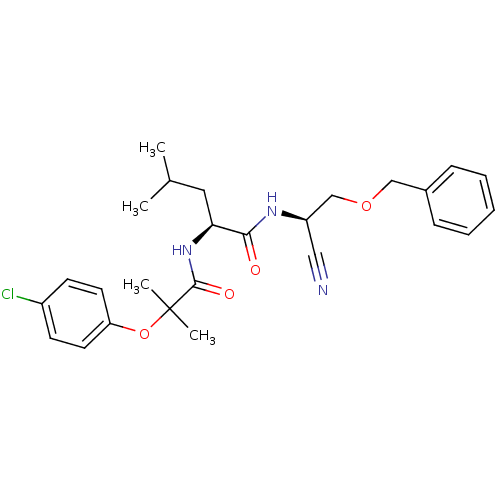 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19759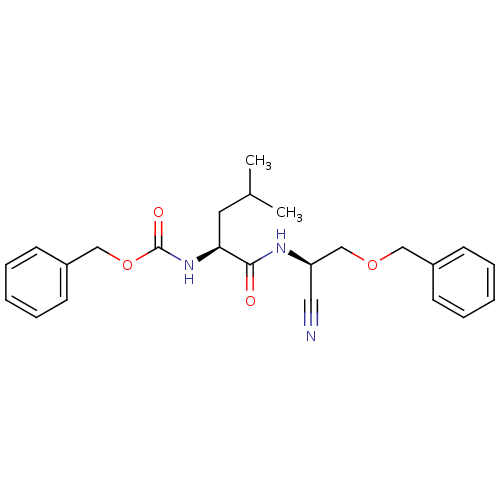 (benzyl N-[(1S)-1-{[(1R)-2-(benzyloxy)-1-cyanoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19761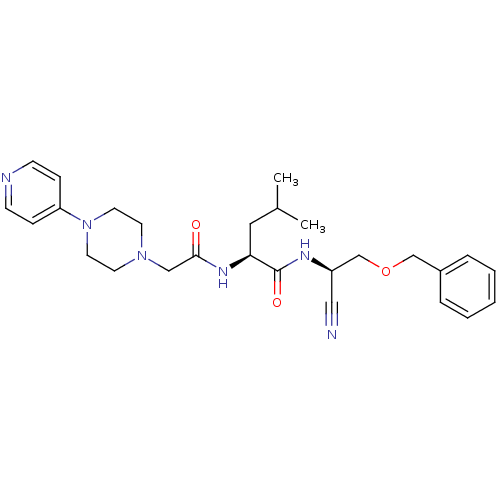 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19766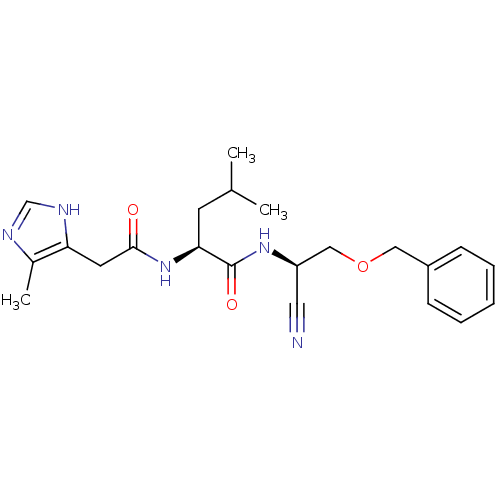 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19764 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-(1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19762 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <30 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19768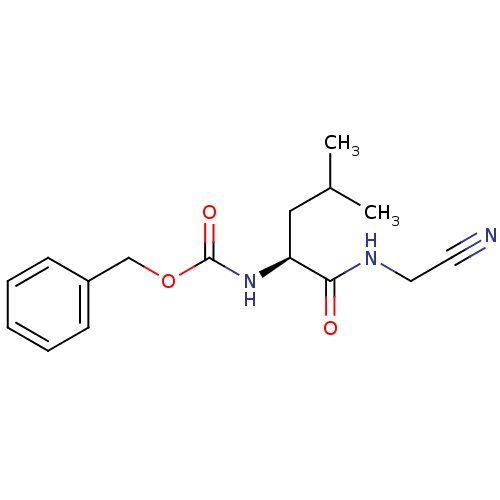 (Cbz-Leu-NH-CH2-CN | JMC487688 Compound 8 | benzyl ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19763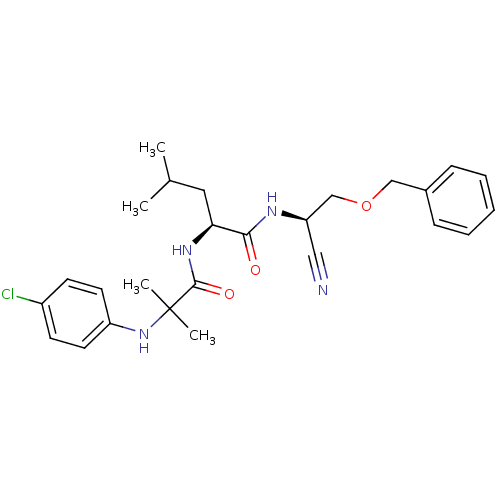 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-{2-[(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19757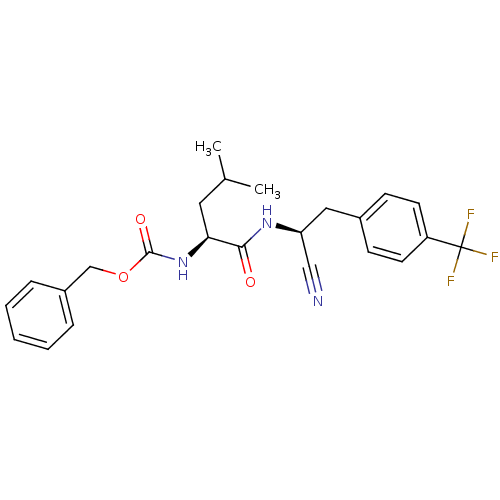 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-[4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19765 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19752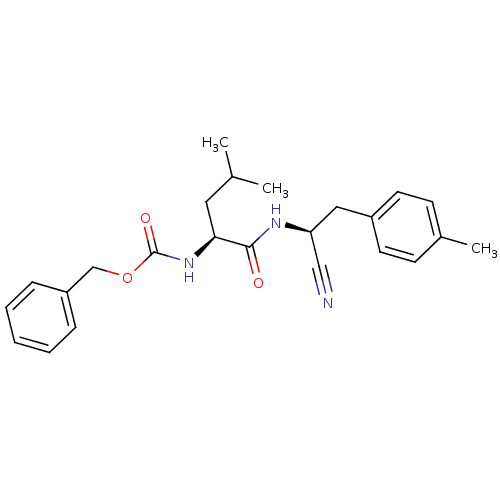 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(4-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19762 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19754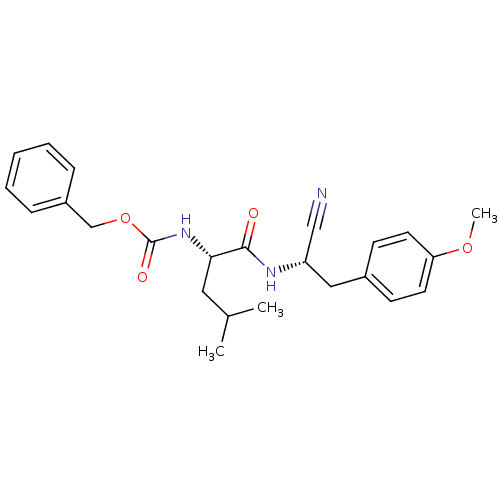 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(4-methoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19765 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19760 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19766 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19753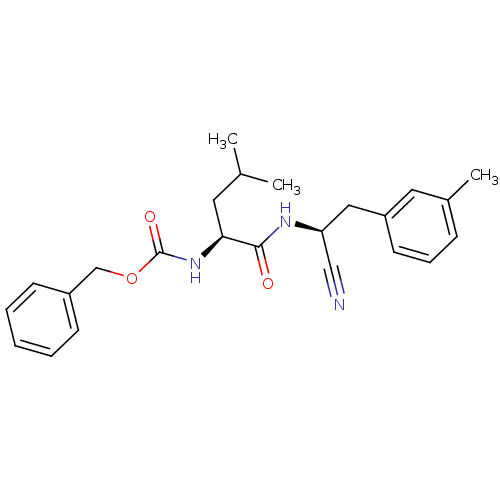 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(3-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19766 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19760 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19764 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-(1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19761 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19767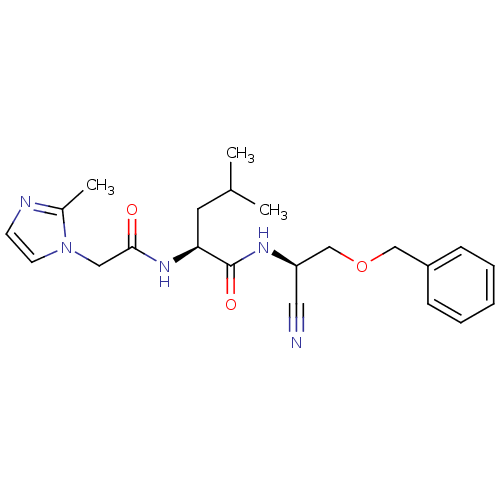 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19763 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-{2-[(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19767 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19758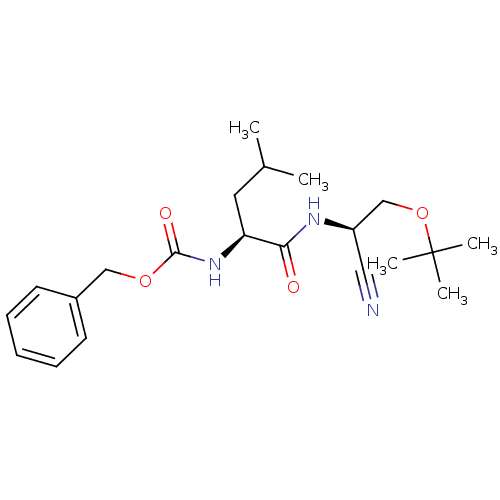 (benzyl N-[(1S)-1-{[(1R)-2-(tert-butoxy)-1-cyanoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19761 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19755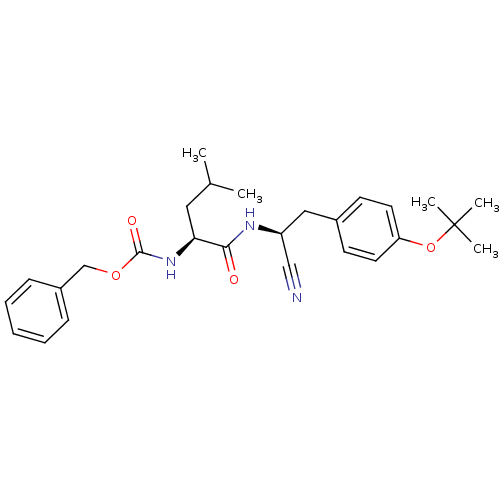 (benzyl N-[(1S)-1-{[(1S)-2-[4-(tert-butoxy)phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19763 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-{2-[(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19767 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19754 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(4-methoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19758 (benzyl N-[(1S)-1-{[(1R)-2-(tert-butoxy)-1-cyanoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19759 (benzyl N-[(1S)-1-{[(1R)-2-(benzyloxy)-1-cyanoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 969 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19756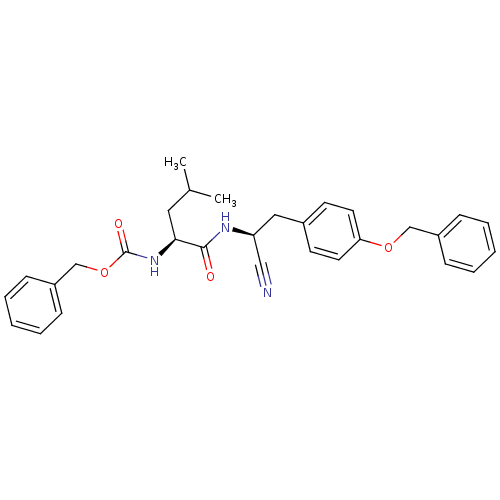 (benzyl N-[(1S)-1-{[(1S)-2-[4-(benzyloxy)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19754 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(4-methoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19760 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19755 (benzyl N-[(1S)-1-{[(1S)-2-[4-(tert-butoxy)phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19759 (benzyl N-[(1S)-1-{[(1R)-2-(benzyloxy)-1-cyanoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19753 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(3-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19758 (benzyl N-[(1S)-1-{[(1R)-2-(tert-butoxy)-1-cyanoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19753 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(3-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19757 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-[4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19752 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(4-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19756 (benzyl N-[(1S)-1-{[(1S)-2-[4-(benzyloxy)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19756 (benzyl N-[(1S)-1-{[(1S)-2-[4-(benzyloxy)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19757 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-[4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19755 (benzyl N-[(1S)-1-{[(1S)-2-[4-(tert-butoxy)phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19752 (benzyl N-[(1S)-1-{[(1S)-1-cyano-2-(4-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||