

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206229 (3-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218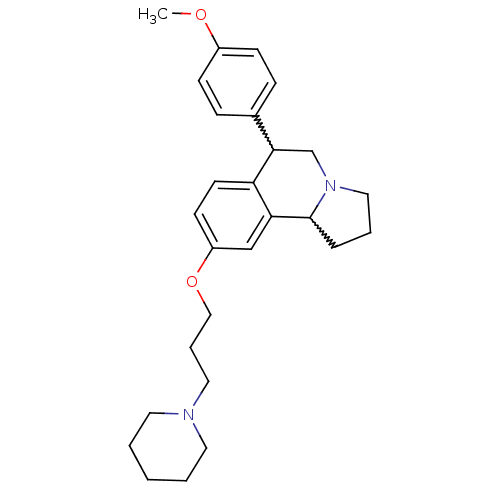 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206222 (6-phenyl-9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206228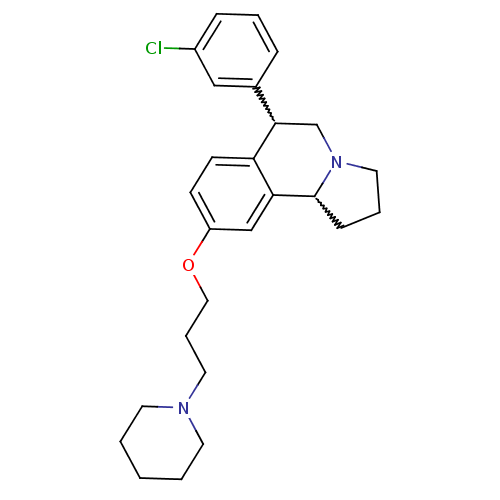 (6-(3-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206235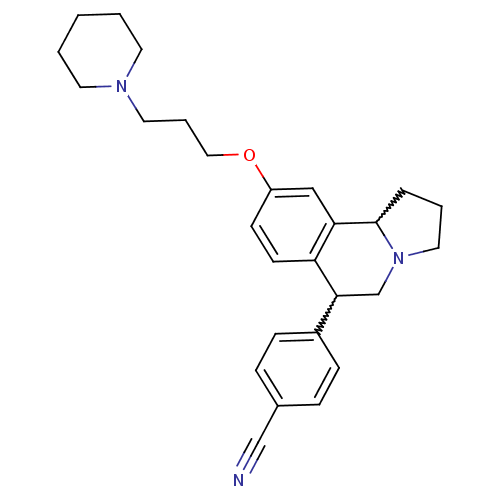 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206225 (6-(4-nitrophenyl)-9-(3-(piperidin-1-yl)propoxy)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206229 (3-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206227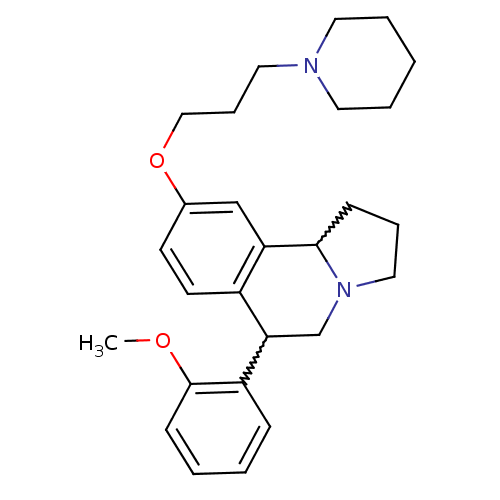 (6-(2-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206233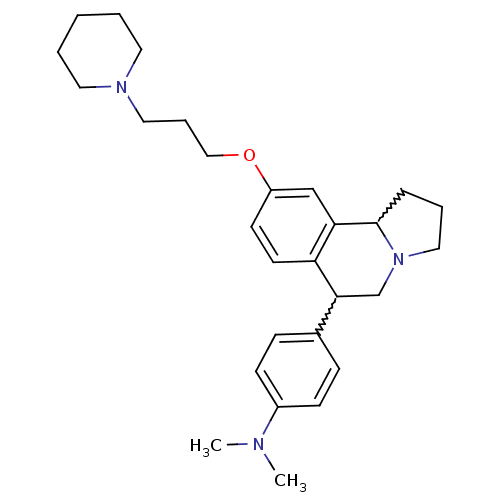 (CHEMBL238951 | N,N-dimethyl-4-(9-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206236 (6-(4-fluorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206221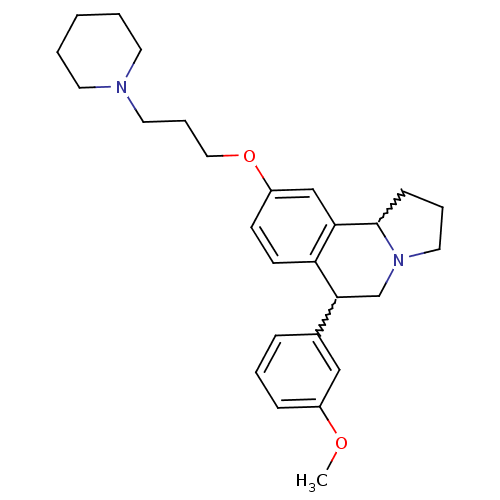 (6-(3-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206217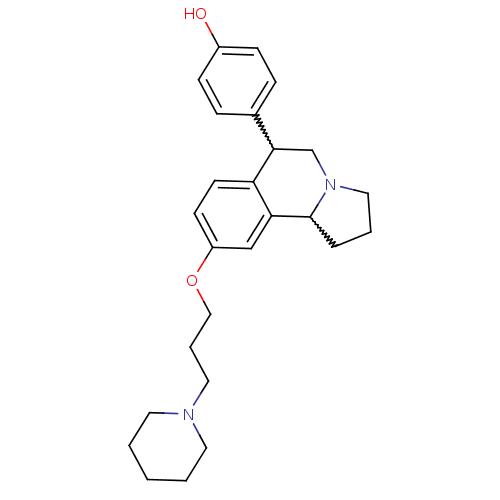 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206219 (9-(3-(piperidin-1-yl)propoxy)-6-(3-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206224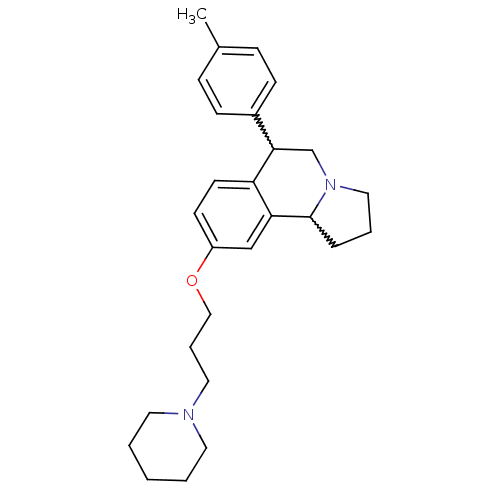 (9-(3-(piperidin-1-yl)propoxy)-6-p-tolyl-1,2,3,5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206232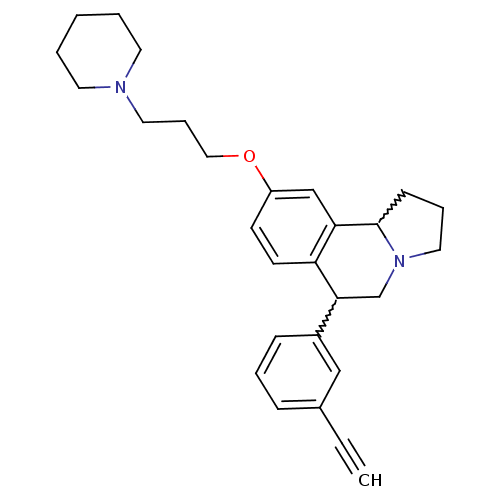 (6-(3-ethynylphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206222 (6-phenyl-9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206237 (6-(4-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206231 (6-(2-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206226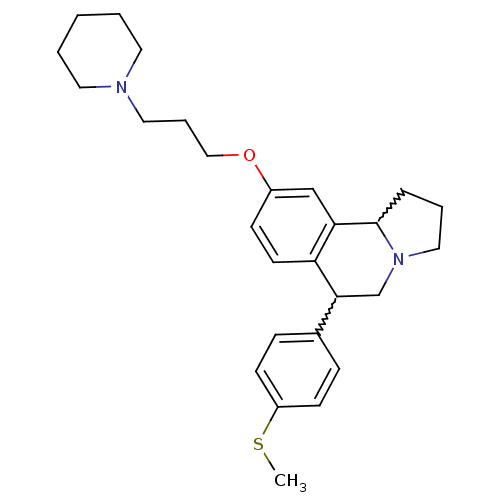 (6-(4-(methylthio)phenyl)-9-(3-(piperidin-1-yl)prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206224 (9-(3-(piperidin-1-yl)propoxy)-6-p-tolyl-1,2,3,5,6,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206226 (6-(4-(methylthio)phenyl)-9-(3-(piperidin-1-yl)prop...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206233 (CHEMBL238951 | N,N-dimethyl-4-(9-(3-(piperidin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206220 (2-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206236 (6-(4-fluorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206228 (6-(3-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206230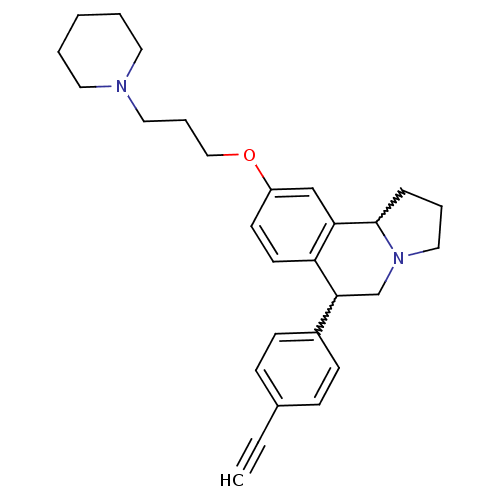 (6-(4-ethynylphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206223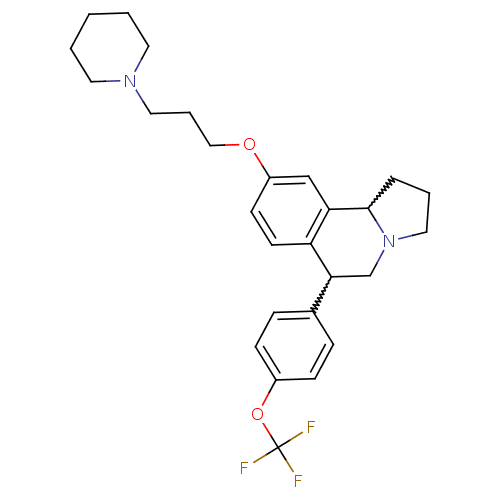 (9-(3-(piperidin-1-yl)propoxy)-6-(4-(trifluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206222 (6-phenyl-9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206236 (6-(4-fluorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206221 (6-(3-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206228 (6-(3-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206224 (9-(3-(piperidin-1-yl)propoxy)-6-p-tolyl-1,2,3,5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206234 (9-(3-(piperidin-1-yl)propoxy)-6-(4-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206226 (6-(4-(methylthio)phenyl)-9-(3-(piperidin-1-yl)prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206225 (6-(4-nitrophenyl)-9-(3-(piperidin-1-yl)propoxy)-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206229 (3-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206233 (CHEMBL238951 | N,N-dimethyl-4-(9-(3-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206237 (6-(4-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206225 (6-(4-nitrophenyl)-9-(3-(piperidin-1-yl)propoxy)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206230 (6-(4-ethynylphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206237 (6-(4-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206221 (6-(3-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206223 (9-(3-(piperidin-1-yl)propoxy)-6-(4-(trifluorometho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206232 (6-(3-ethynylphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206231 (6-(2-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206217 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206231 (6-(2-chlorophenyl)-9-(3-(piperidin-1-yl)propoxy)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206223 (9-(3-(piperidin-1-yl)propoxy)-6-(4-(trifluorometho...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206227 (6-(2-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206232 (6-(3-ethynylphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206230 (6-(4-ethynylphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206220 (2-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206219 (9-(3-(piperidin-1-yl)propoxy)-6-(3-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206219 (9-(3-(piperidin-1-yl)propoxy)-6-(3-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206235 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50206234 (9-(3-(piperidin-1-yl)propoxy)-6-(4-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain SERT | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206227 (6-(2-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206234 (9-(3-(piperidin-1-yl)propoxy)-6-(4-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206235 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206217 (4-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206220 (2-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50206218 (6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl)propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of N-[3H]-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells | Bioorg Med Chem Lett 17: 2603-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.106 BindingDB Entry DOI: 10.7270/Q2HH6JR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||