 Found 11 hits of Enzyme Inhibition Constant Data
Found 11 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50216551
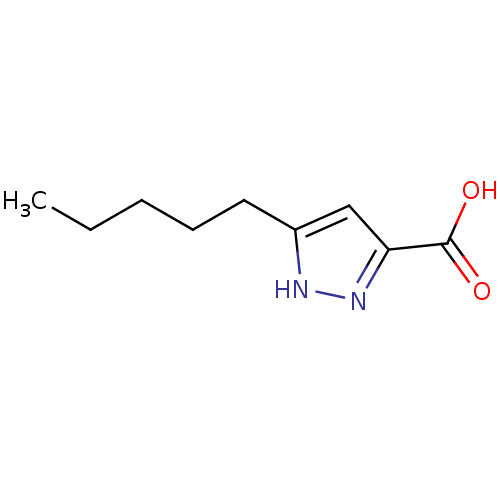
(3-pentyl-1H-pyrazole-5-carboxylic acid | 5-butylpy...)Show InChI InChI=1S/C9H14N2O2/c1-2-3-4-5-7-6-8(9(12)13)11-10-7/h6H,2-5H2,1H3,(H,10,11)(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at GPR109a |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50132140
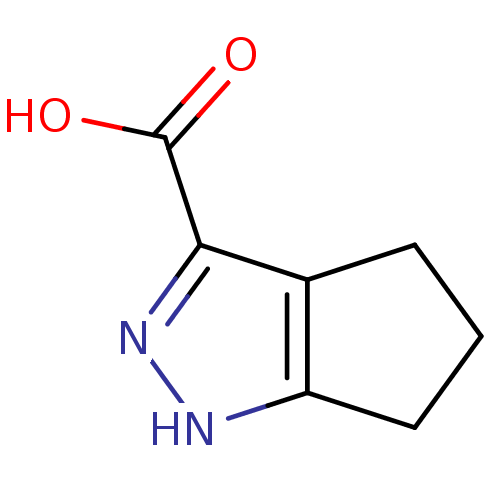
(1,4,5,6-Tetrahydro-cyclopentapyrazole-3-carboxylic...)Show InChI InChI=1S/C7H8N2O2/c10-7(11)6-4-2-1-3-5(4)8-9-6/h1-3H2,(H,8,9)(H,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at GPR109a |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50132142
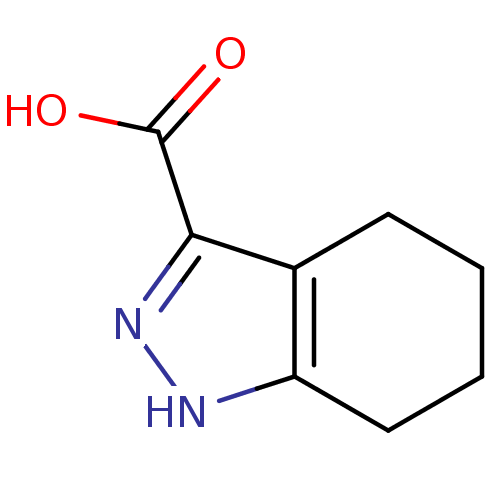
(4,5,6,7-Tetrahydro-1H-indazole-3-carboxylic acid |...)Show InChI InChI=1S/C8H10N2O2/c11-8(12)7-5-3-1-2-4-6(5)9-10-7/h1-4H2,(H,9,10)(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at GPR109a |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50208138
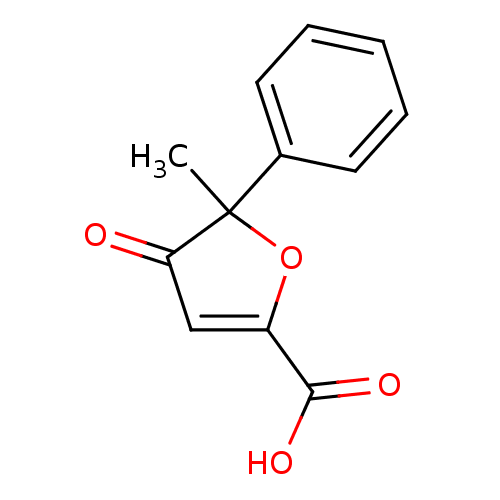
((+)-acifran | (-)-acifran | 5-Methyl-4-oxo-5-pheny...)Show InChI InChI=1S/C12H10O4/c1-12(8-5-3-2-4-6-8)10(13)7-9(16-12)11(14)15/h2-7H,1H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109a (unknown origin) assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50208138

((+)-acifran | (-)-acifran | 5-Methyl-4-oxo-5-pheny...)Show InChI InChI=1S/C12H10O4/c1-12(8-5-3-2-4-6-8)10(13)7-9(16-12)11(14)15/h2-7H,1H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109a (unknown origin) assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208138

((+)-acifran | (-)-acifran | 5-Methyl-4-oxo-5-pheny...)Show InChI InChI=1S/C12H10O4/c1-12(8-5-3-2-4-6-8)10(13)7-9(16-12)11(14)15/h2-7H,1H3,(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109b assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50241028
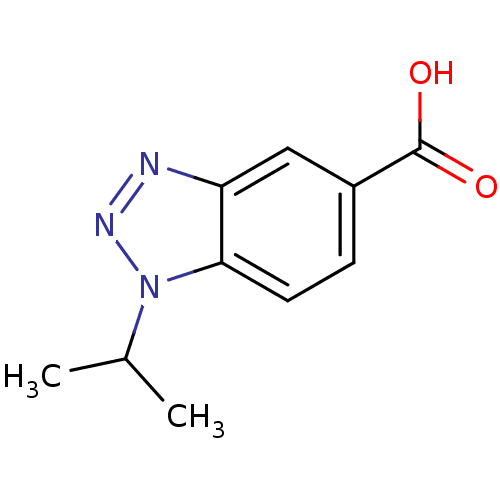
(1-Isopropyl-1H-benzotriazole-5-carboxylic acid | 1...)Show InChI InChI=1S/C10H11N3O2/c1-6(2)13-9-4-3-7(10(14)15)5-8(9)11-12-13/h3-6H,1-2H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109a (unknown origin) assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50208122
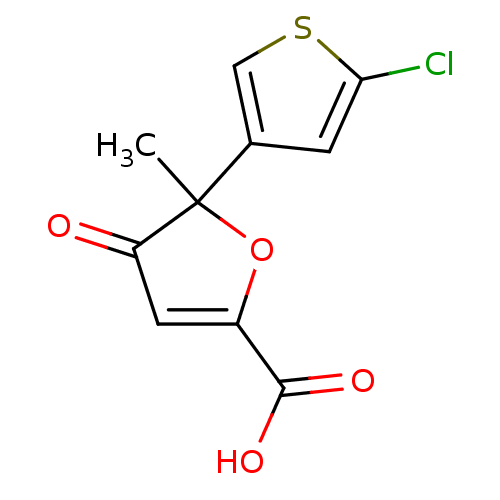
((+)-5-(5-chloro-thiophen-3-yl)-5-methyl-4-oxo-4,5-...)Show InChI InChI=1S/C10H7ClO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109a (unknown origin) assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208122

((+)-5-(5-chloro-thiophen-3-yl)-5-methyl-4-oxo-4,5-...)Show InChI InChI=1S/C10H7ClO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109b assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50208137
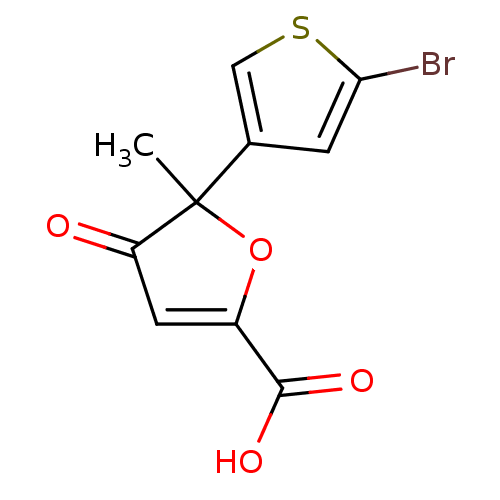
((+)-5-(5-bromo-thiophen-3-yl)-5-methyl-4-oxo-4,5-d...)Show InChI InChI=1S/C10H7BrO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109b assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50208137

((+)-5-(5-bromo-thiophen-3-yl)-5-methyl-4-oxo-4,5-d...)Show InChI InChI=1S/C10H7BrO4S/c1-10(5-2-8(11)16-4-5)7(12)3-6(15-10)9(13)14/h2-4H,1H3,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity at GPR109a (unknown origin) assessed as inhibition of forskolin-stimulated cAMP production |
J Med Chem 51: 7653-62 (2008)
Article DOI: 10.1021/jm800896z
BindingDB Entry DOI: 10.7270/Q27D2TZ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data