

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300312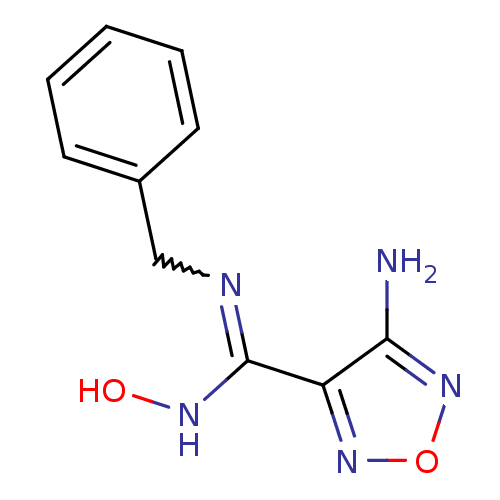 (4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300306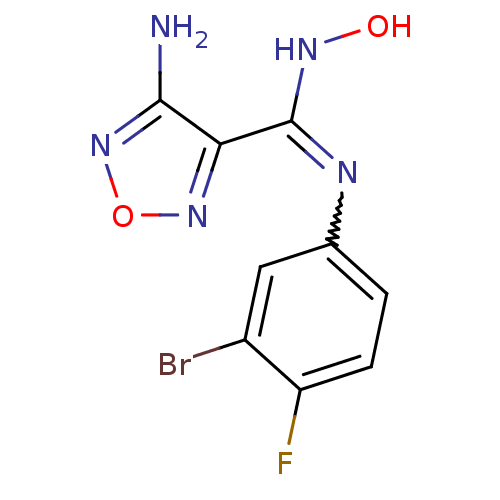 (4-Amino-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300299 (4-Amino-N-(3-bromophenyl)-N'-hydroxy-1,2,5-oxadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300296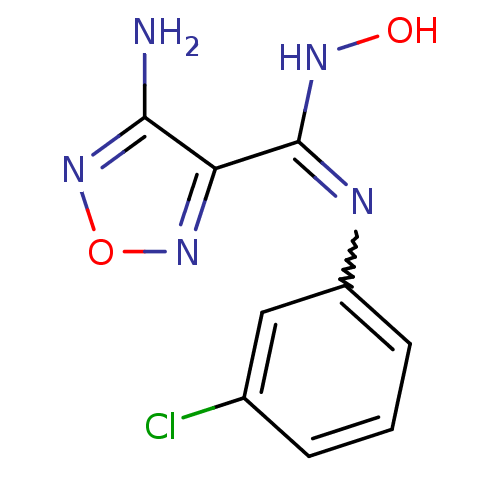 (4-Amino-N-(3-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300305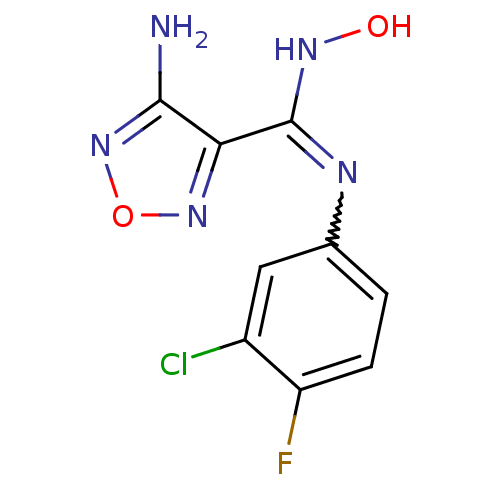 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in mouse B16 cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300301 (4-Amino-N-(3-ethylphenyl)-N'-hydroxy-1,2,5-oxadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300306 (4-Amino-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300299 (4-Amino-N-(3-bromophenyl)-N'-hydroxy-1,2,5-oxadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300296 (4-Amino-N-(3-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300300 (4-Amino-N'-hydroxy-N-(3-methylphenyl)-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300302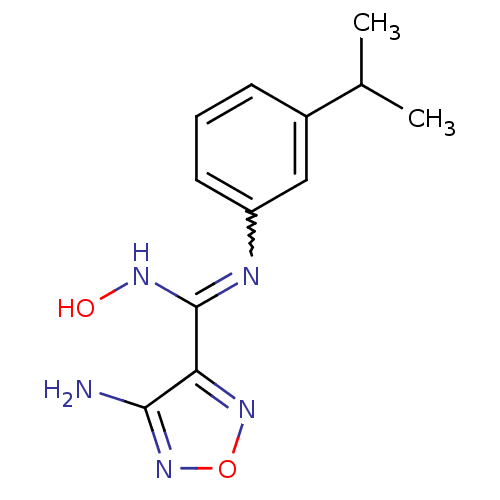 (4-Amino-N'-hydroxy-N-(3-isopropylphenyl)-1,2,5-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300298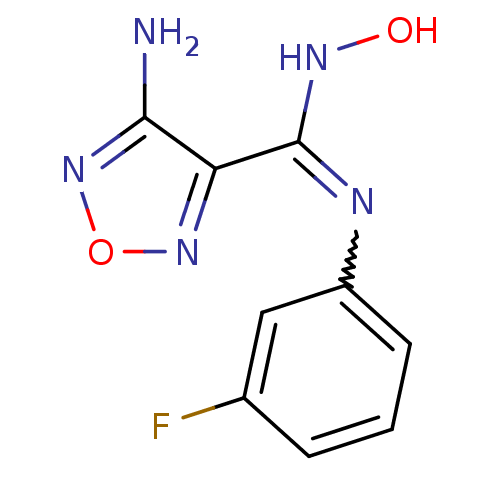 (4-Amino-N-(3-fluorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300301 (4-Amino-N-(3-ethylphenyl)-N'-hydroxy-1,2,5-oxadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300294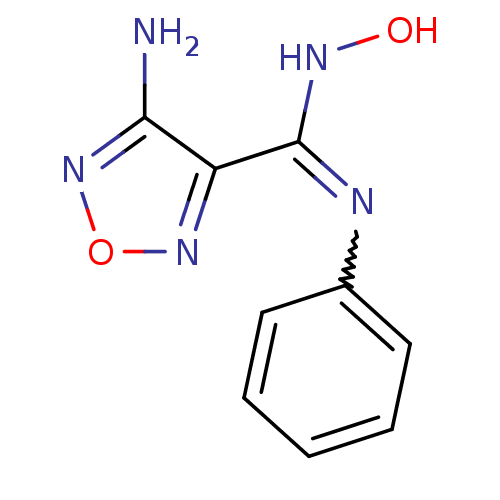 (4-Amino-N'-hydroxy-N-phenyl-1,2,5-oxadiazole-3-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300298 (4-Amino-N-(3-fluorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300300 (4-Amino-N'-hydroxy-N-(3-methylphenyl)-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300304 (4-Amino-N'-hydroxy-N-(3-methoxyphenyl)-1,2,5-oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300312 (4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in mouse B16 cells assessed as kynurenine formation by adjusted cell-based spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300309 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300295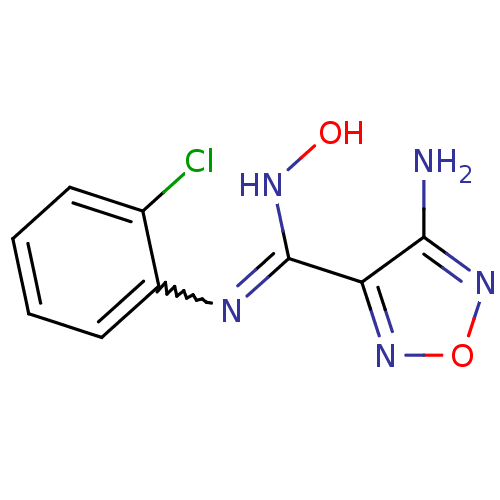 (4-Amino-N-(2-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300311 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300309 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300310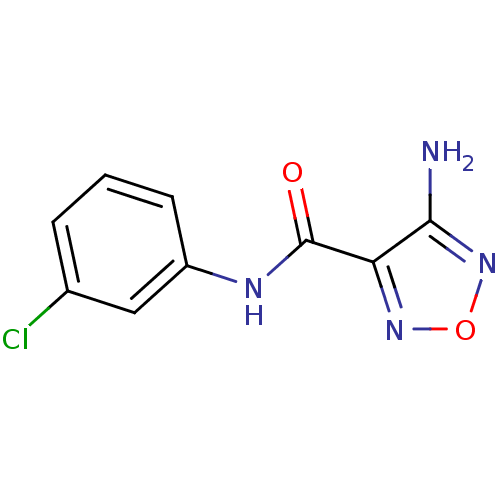 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300307 (4-Aamino-N-(3-chlorophenyl)-N'-methoxy-1,2,5-oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300308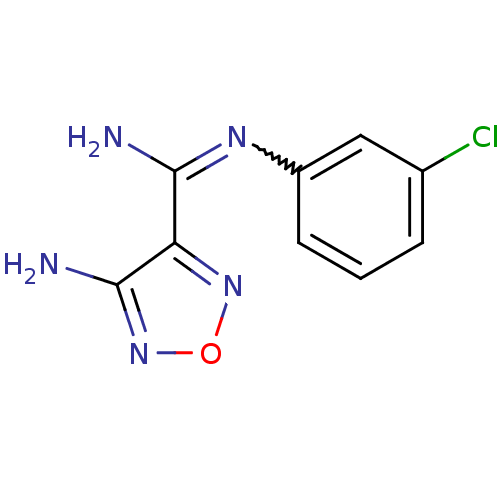 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300302 (4-Amino-N'-hydroxy-N-(3-isopropylphenyl)-1,2,5-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300297 (4-Amino-N-(4-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300294 (4-Amino-N'-hydroxy-N-phenyl-1,2,5-oxadiazole-3-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300304 (4-Amino-N'-hydroxy-N-(3-methoxyphenyl)-1,2,5-oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300297 (4-Amino-N-(4-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300295 (4-Amino-N-(2-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300303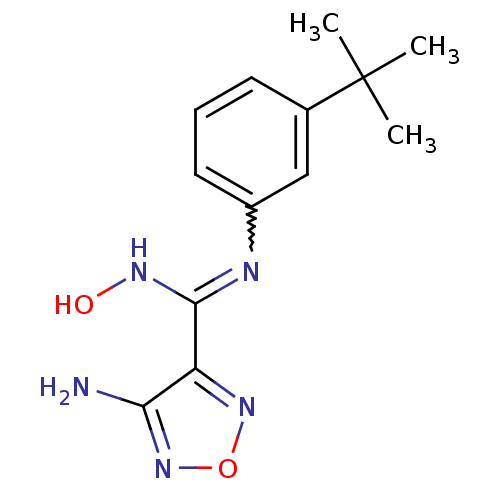 (4-Amino-N-(3-tert-butylphenyl)-N'-hydroxy-1,2,5-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of tryptophan 2,3-dioxygenase | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of tryptophan 2,3-dioxygenase by cell-based assay | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300311 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300308 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300307 (4-Aamino-N-(3-chlorophenyl)-N'-methoxy-1,2,5-oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300310 (4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300303 (4-Amino-N-(3-tert-butylphenyl)-N'-hydroxy-1,2,5-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation Curated by ChEMBL | Assay Description Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... | J Med Chem 52: 7364-7 (2009) Article DOI: 10.1021/jm900518f BindingDB Entry DOI: 10.7270/Q29P32KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||