 Found 41 hits of Enzyme Inhibition Constant Data
Found 41 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314290
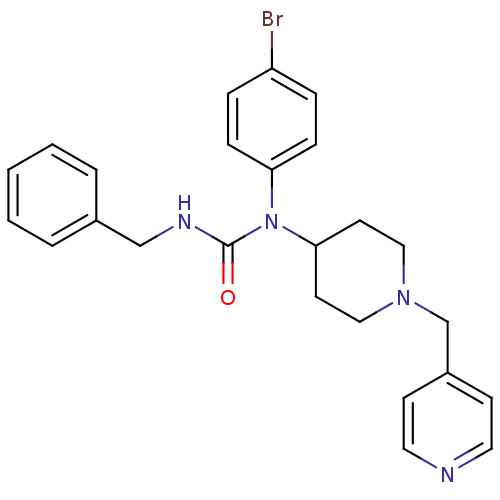
(3-benzyl-1-(4-bromophenyl)-1-(1-(pyridin-4-ylmethy...)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccncc2)CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H27BrN4O/c26-22-6-8-23(9-7-22)30(25(31)28-18-20-4-2-1-3-5-20)24-12-16-29(17-13-24)19-21-10-14-27-15-11-21/h1-11,14-15,24H,12-13,16-19H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314288
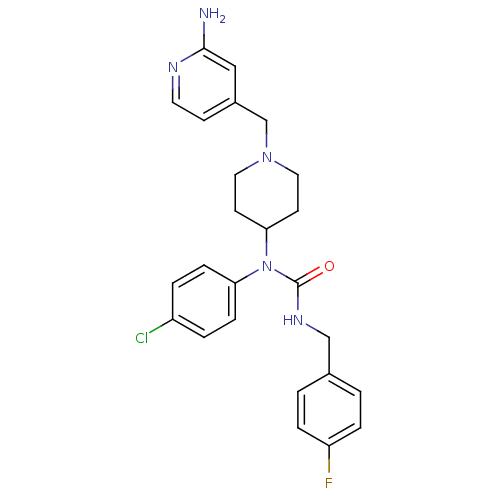
(1-(1-((2-aminopyridin-4-yl)methyl)piperidin-4-yl)-...)Show SMILES Nc1cc(CN2CCC(CC2)N(C(=O)NCc2ccc(F)cc2)c2ccc(Cl)cc2)ccn1 Show InChI InChI=1S/C25H27ClFN5O/c26-20-3-7-22(8-4-20)32(25(33)30-16-18-1-5-21(27)6-2-18)23-10-13-31(14-11-23)17-19-9-12-29-24(28)15-19/h1-9,12,15,23H,10-11,13-14,16-17H2,(H2,28,29)(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314296

(1-(4-chlorophenyl)-1-(1-ethylpiperidin-4-yl)-3-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClFN3O/c1-2-25-13-11-20(12-14-25)26(19-9-5-17(22)6-10-19)21(27)24-15-16-3-7-18(23)8-4-16/h3-10,20H,2,11-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314294
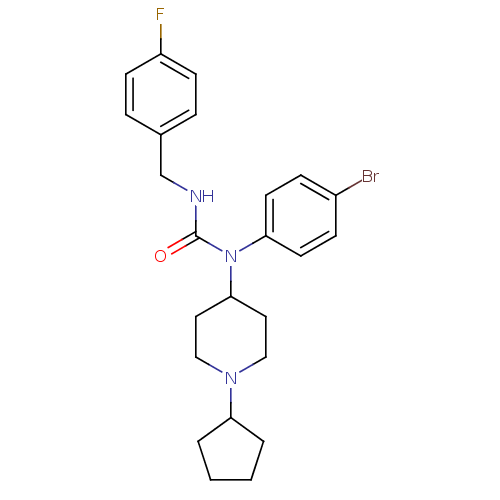
(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H29BrFN3O/c25-19-7-11-22(12-8-19)29(24(30)27-17-18-5-9-20(26)10-6-18)23-13-15-28(16-14-23)21-3-1-2-4-21/h5-12,21,23H,1-4,13-17H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314291
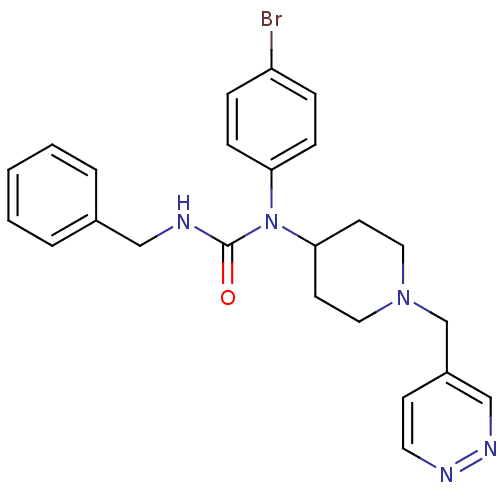
(3-benzyl-1-(4-bromophenyl)-1-(1-(pyridazin-4-ylmet...)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccnnc2)CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H26BrN5O/c25-21-6-8-22(9-7-21)30(24(31)26-16-19-4-2-1-3-5-19)23-11-14-29(15-12-23)18-20-10-13-27-28-17-20/h1-10,13,17,23H,11-12,14-16,18H2,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314292
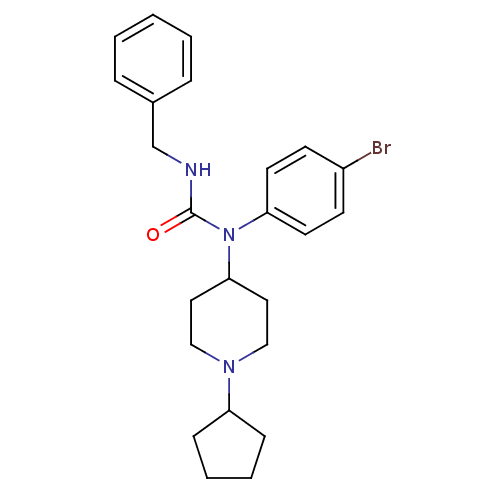
(3-benzyl-1-(4-bromophenyl)-1-(1-cyclopentylpiperid...)Show SMILES Brc1ccc(cc1)N(C1CCN(CC1)C1CCCC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H30BrN3O/c25-20-10-12-22(13-11-20)28(24(29)26-18-19-6-2-1-3-7-19)23-14-16-27(17-15-23)21-8-4-5-9-21/h1-3,6-7,10-13,21,23H,4-5,8-9,14-18H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314293

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Brc1ccc(cc1)N(C1CCN(CC1)C1CCCC1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C23H28BrN3O/c24-18-10-12-21(13-11-18)27(23(28)25-19-6-2-1-3-7-19)22-14-16-26(17-15-22)20-8-4-5-9-20/h1-3,6-7,10-13,20,22H,4-5,8-9,14-17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314311

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyridi...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccncc3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O/c26-21-3-7-23(8-4-21)31(25(32)29-17-19-1-5-22(27)6-2-19)24-11-15-30(16-12-24)18-20-9-13-28-14-10-20/h1-10,13-14,24H,11-12,15-18H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314295
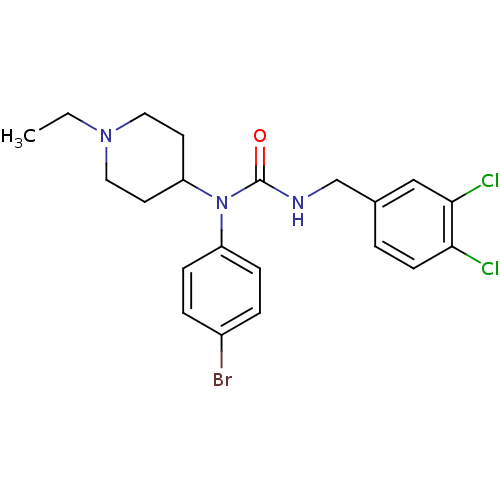
(1-(4-bromophenyl)-3-(3,4-dichlorobenzyl)-1-(1-ethy...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(Cl)c(Cl)c1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H24BrCl2N3O/c1-2-26-11-9-18(10-12-26)27(17-6-4-16(22)5-7-17)21(28)25-14-15-3-8-19(23)20(24)13-15/h3-8,13,18H,2,9-12,14H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314313

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Clc1cc(Cl)cc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)c1 Show InChI InChI=1S/C24H28BrCl2N3O/c25-18-5-7-22(8-6-18)30(23-9-11-29(12-10-23)21-3-1-2-4-21)24(31)28-16-17-13-19(26)15-20(27)14-17/h5-8,13-15,21,23H,1-4,9-12,16H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314297

(1-(5-chloropyridin-2-yl)-1-(1-ethylpiperidin-4-yl)...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cn1 Show InChI InChI=1S/C20H24ClFN4O/c1-2-25-11-9-18(10-12-25)26(19-8-5-16(21)14-23-19)20(27)24-13-15-3-6-17(22)7-4-15/h3-8,14,18H,2,9-13H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314315

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES COc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C25H32BrN3O2/c1-31-24-12-6-19(7-13-24)18-27-25(30)29(22-10-8-20(26)9-11-22)23-14-16-28(17-15-23)21-4-2-3-5-21/h6-13,21,23H,2-5,14-18H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314289

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyrida...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccnnc3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C24H25ClFN5O/c25-20-3-7-22(8-4-20)31(24(32)27-15-18-1-5-21(26)6-2-18)23-10-13-30(14-11-23)17-19-9-12-28-29-16-19/h1-9,12,16,23H,10-11,13-15,17H2,(H,27,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314307
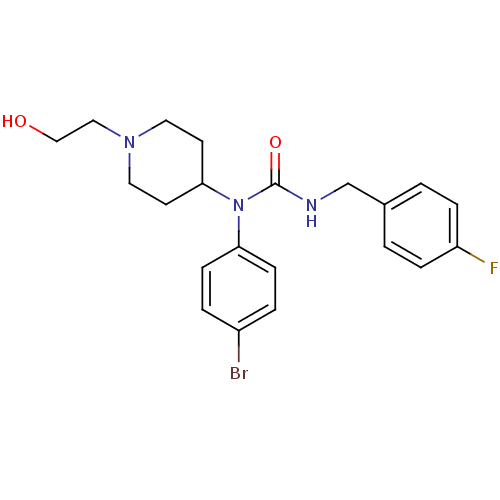
(1-(4-bromophenyl)-3-(4-fluorobenzyl)-1-(1-(2-hydro...)Show SMILES OCCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H25BrFN3O2/c22-17-3-7-19(8-4-17)26(20-9-11-25(12-10-20)13-14-27)21(28)24-15-16-1-5-18(23)6-2-16/h1-8,20,27H,9-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314306
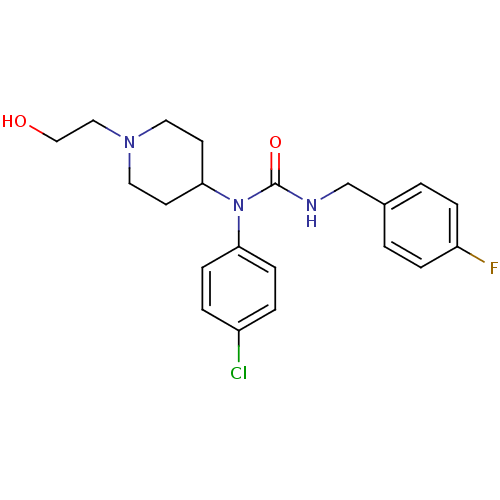
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(2-hydr...)Show SMILES OCCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClFN3O2/c22-17-3-7-19(8-4-17)26(20-9-11-25(12-10-20)13-14-27)21(28)24-15-16-1-5-18(23)6-2-16/h1-8,20,27H,9-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314314

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Clc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1Cl Show InChI InChI=1S/C24H28BrCl2N3O/c25-18-6-8-20(9-7-18)30(21-11-13-29(14-12-21)19-3-1-2-4-19)24(31)28-16-17-5-10-22(26)23(27)15-17/h5-10,15,19,21H,1-4,11-14,16H2,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314298

(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C21H25F2N3O/c1-2-25-13-11-20(12-14-25)26(19-9-7-18(23)8-10-19)21(27)24-15-16-3-5-17(22)6-4-16/h3-10,20H,2,11-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314300
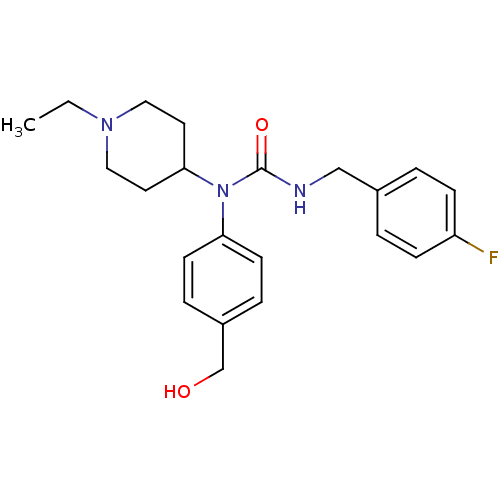
(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(CO)cc1 Show InChI InChI=1S/C22H28FN3O2/c1-2-25-13-11-21(12-14-25)26(20-9-5-18(16-27)6-10-20)22(28)24-15-17-3-7-19(23)8-4-17/h3-10,21,27H,2,11-16H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314312
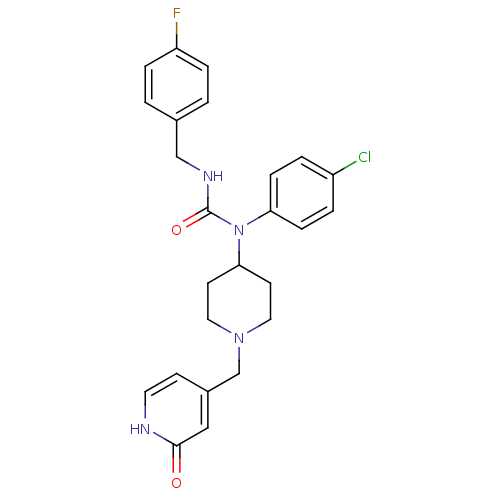
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-((2-hyd...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3cc[nH]c(=O)c3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O2/c26-20-3-7-22(8-4-20)31(25(33)29-16-18-1-5-21(27)6-2-18)23-10-13-30(14-11-23)17-19-9-12-28-24(32)15-19/h1-9,12,15,23H,10-11,13-14,16-17H2,(H,28,32)(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314305

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(methyl...)Show SMILES CS(=O)(=O)N1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H23ClFN3O3S/c1-29(27,28)24-12-10-19(11-13-24)25(18-8-4-16(21)5-9-18)20(26)23-14-15-2-6-17(22)7-3-15/h2-9,19H,10-14H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314310
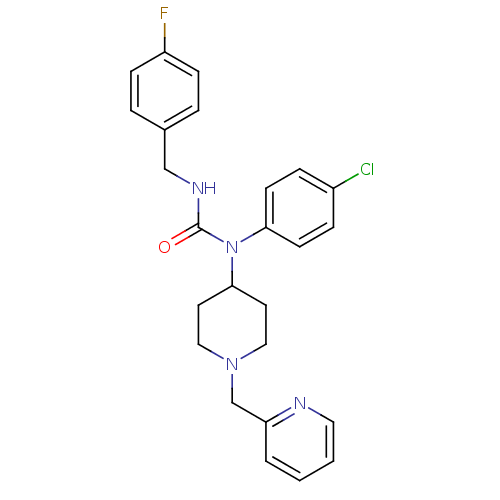
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyridi...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccccn3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O/c26-20-6-10-23(11-7-20)31(25(32)29-17-19-4-8-21(27)9-5-19)24-12-15-30(16-13-24)18-22-3-1-2-14-28-22/h1-11,14,24H,12-13,15-18H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314302

(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(pi...)Show InChI InChI=1S/C20H31FN4O/c1-2-24-13-9-19(10-14-24)25(18-7-11-22-12-8-18)20(26)23-15-16-3-5-17(21)6-4-16/h3-6,18-19,22H,2,7-15H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314308

(1-(4-chlorophenyl)-1-(1-(2,3-dihydroxypropyl)piper...)Show SMILES OCC(O)CN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H27ClFN3O3/c23-17-3-7-19(8-4-17)27(20-9-11-26(12-10-20)14-21(29)15-28)22(30)25-13-16-1-5-18(24)6-2-16/h1-8,20-21,28-29H,9-15H2,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314299
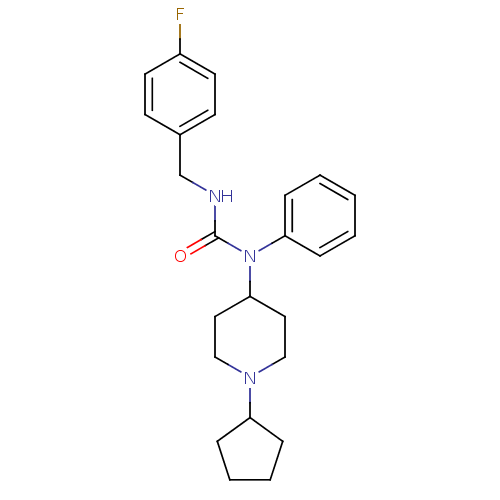
(1-(1-cyclopentylpiperidin-4-yl)-3-(4-fluorobenzyl)...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccccc2)cc1 Show InChI InChI=1S/C24H30FN3O/c25-20-12-10-19(11-13-20)18-26-24(29)28(22-8-2-1-3-9-22)23-14-16-27(17-15-23)21-6-4-5-7-21/h1-3,8-13,21,23H,4-7,14-18H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314303

(1-(1-ethylpiperidin-4-yl)-3-(4-fluorobenzyl)-1-(1-...)Show SMILES CCN1CCC(CC1)N(C1CCN(CCO)CC1)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C22H35FN4O2/c1-2-25-11-7-20(8-12-25)27(21-9-13-26(14-10-21)15-16-28)22(29)24-17-18-3-5-19(23)6-4-18/h3-6,20-21,28H,2,7-17H2,1H3,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314309

(4-(1-(4-chlorophenyl)-3-(4-fluorobenzyl)ureido)-N-...)Show SMILES CCNC(=O)N1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H26ClFN4O2/c1-2-25-21(29)27-13-11-20(12-14-27)28(19-9-5-17(23)6-10-19)22(30)26-15-16-3-7-18(24)8-4-16/h3-10,20H,2,11-15H2,1H3,(H,25,29)(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314301
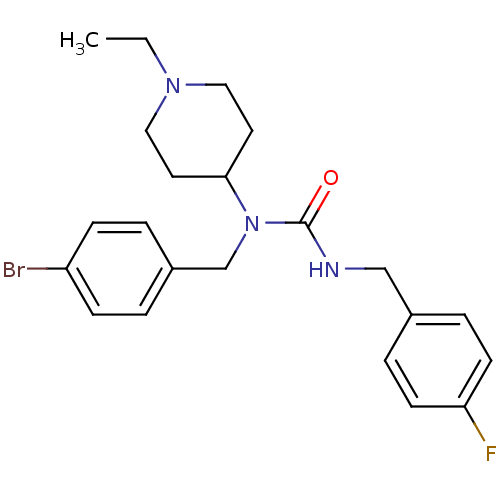
(1-(4-bromobenzyl)-1-(1-ethylpiperidin-4-yl)-3-(4-f...)Show SMILES CCN1CCC(CC1)N(Cc1ccc(Br)cc1)C(=O)NCc1ccc(F)cc1 Show InChI InChI=1S/C22H27BrFN3O/c1-2-26-13-11-21(12-14-26)27(16-18-3-7-19(23)8-4-18)22(28)25-15-17-5-9-20(24)10-6-17/h3-10,21H,2,11-16H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50314304
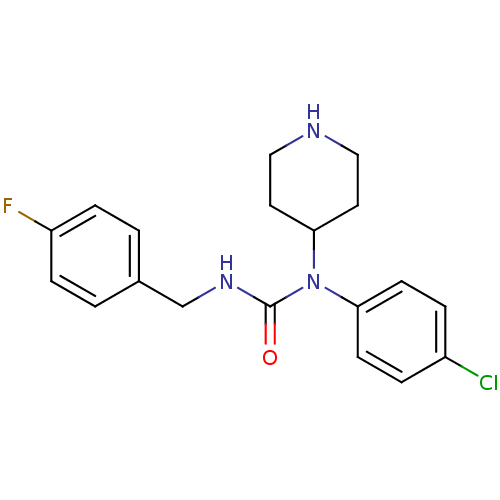
(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(piperidin...)Show InChI InChI=1S/C19H21ClFN3O/c20-15-3-7-17(8-4-15)24(18-9-11-22-12-10-18)19(25)23-13-14-1-5-16(21)6-2-14/h1-8,18,22H,9-13H2,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine 3 receptor |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314313

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Clc1cc(Cl)cc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)c1 Show InChI InChI=1S/C24H28BrCl2N3O/c25-18-5-7-22(8-6-18)30(23-9-11-29(12-10-23)21-3-1-2-4-21)24(31)28-16-17-13-19(26)15-20(27)14-17/h5-8,13-15,21,23H,1-4,9-12,16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314292

(3-benzyl-1-(4-bromophenyl)-1-(1-cyclopentylpiperid...)Show SMILES Brc1ccc(cc1)N(C1CCN(CC1)C1CCCC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H30BrN3O/c25-20-10-12-22(13-11-20)28(24(29)26-18-19-6-2-1-3-7-19)23-14-16-27(17-15-23)21-8-4-5-9-21/h1-3,6-7,10-13,21,23H,4-5,8-9,14-18H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314293

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Brc1ccc(cc1)N(C1CCN(CC1)C1CCCC1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C23H28BrN3O/c24-18-10-12-21(13-11-18)27(23(28)25-19-6-2-1-3-7-19)22-14-16-26(17-15-22)20-8-4-5-9-20/h1-3,6-7,10-13,20,22H,4-5,8-9,14-17H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314294

(1-(4-bromophenyl)-1-(1-cyclopentylpiperidin-4-yl)-...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(CC2)C2CCCC2)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H29BrFN3O/c25-19-7-11-22(12-8-19)29(24(30)27-17-18-5-9-20(26)10-6-18)23-13-15-28(16-14-23)21-3-1-2-4-21/h5-12,21,23H,1-4,13-17H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314288

(1-(1-((2-aminopyridin-4-yl)methyl)piperidin-4-yl)-...)Show SMILES Nc1cc(CN2CCC(CC2)N(C(=O)NCc2ccc(F)cc2)c2ccc(Cl)cc2)ccn1 Show InChI InChI=1S/C25H27ClFN5O/c26-20-3-7-22(8-4-20)32(25(33)30-16-18-1-5-21(27)6-2-18)23-10-13-31(14-11-23)17-19-9-12-29-24(28)15-19/h1-9,12,15,23H,10-11,13-14,16-17H2,(H2,28,29)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314290

(3-benzyl-1-(4-bromophenyl)-1-(1-(pyridin-4-ylmethy...)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccncc2)CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H27BrN4O/c26-22-6-8-23(9-7-22)30(25(31)28-18-20-4-2-1-3-5-20)24-12-16-29(17-13-24)19-21-10-14-27-15-11-21/h1-11,14-15,24H,12-13,16-19H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314311

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyridi...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccncc3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H26ClFN4O/c26-21-3-7-23(8-4-21)31(25(32)29-17-19-1-5-22(27)6-2-19)24-11-15-30(16-12-24)18-20-9-13-28-14-10-20/h1-10,13-14,24H,11-12,15-18H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314296

(1-(4-chlorophenyl)-1-(1-ethylpiperidin-4-yl)-3-(4-...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClFN3O/c1-2-25-13-11-20(12-14-25)26(19-9-5-17(22)6-10-19)21(27)24-15-16-3-7-18(23)8-4-16/h3-10,20H,2,11-15H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314307

(1-(4-bromophenyl)-3-(4-fluorobenzyl)-1-(1-(2-hydro...)Show SMILES OCCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Br)cc1 Show InChI InChI=1S/C21H25BrFN3O2/c22-17-3-7-19(8-4-17)26(20-9-11-25(12-10-20)13-14-27)21(28)24-15-16-1-5-18(23)6-2-16/h1-8,20,27H,9-15H2,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314297

(1-(5-chloropyridin-2-yl)-1-(1-ethylpiperidin-4-yl)...)Show SMILES CCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cn1 Show InChI InChI=1S/C20H24ClFN4O/c1-2-25-11-9-18(10-12-25)26(19-8-5-16(21)14-23-19)20(27)24-13-15-3-6-17(22)7-4-15/h3-8,14,18H,2,9-13H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314289

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(pyrida...)Show SMILES Fc1ccc(CNC(=O)N(C2CCN(Cc3ccnnc3)CC2)c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C24H25ClFN5O/c25-20-3-7-22(8-4-20)31(24(32)27-15-18-1-5-21(26)6-2-18)23-10-13-30(14-11-23)17-19-9-12-28-29-16-19/h1-9,12,16,23H,10-11,13-15,17H2,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314306

(1-(4-chlorophenyl)-3-(4-fluorobenzyl)-1-(1-(2-hydr...)Show SMILES OCCN1CCC(CC1)N(C(=O)NCc1ccc(F)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClFN3O2/c22-17-3-7-19(8-4-17)26(20-9-11-25(12-10-20)13-14-27)21(28)24-15-16-1-5-18(23)6-2-16/h1-8,20,27H,9-15H2,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314291

(3-benzyl-1-(4-bromophenyl)-1-(1-(pyridazin-4-ylmet...)Show SMILES Brc1ccc(cc1)N(C1CCN(Cc2ccnnc2)CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H26BrN5O/c25-21-6-8-22(9-7-21)30(24(31)26-16-19-4-2-1-3-5-19)23-11-14-29(15-12-23)18-20-10-13-27-28-17-20/h1-10,13,17,23H,11-12,14-16,18H2,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by ion works assay |
Bioorg Med Chem Lett 20: 2359-64 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.121
BindingDB Entry DOI: 10.7270/Q2Z31ZSM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data