

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960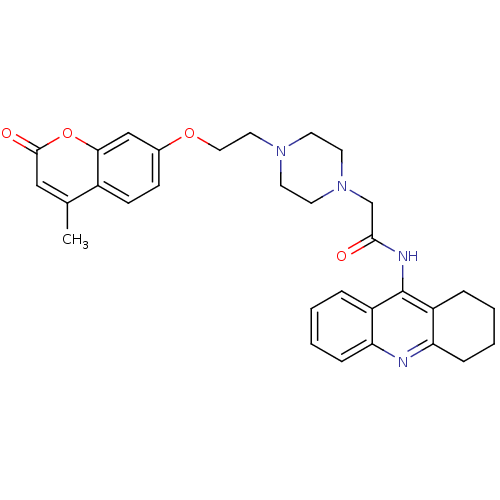 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435960 (CHEMBL2391486) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435956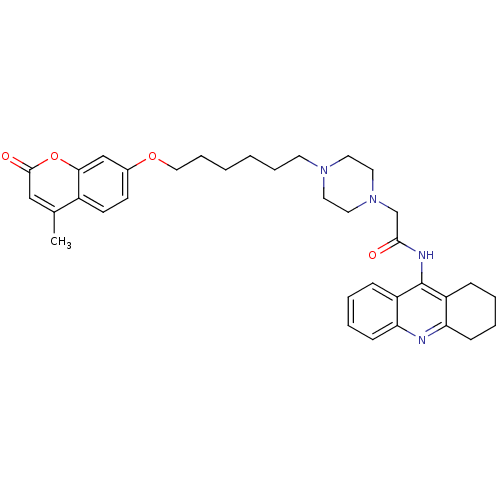 (CHEMBL2391490) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435953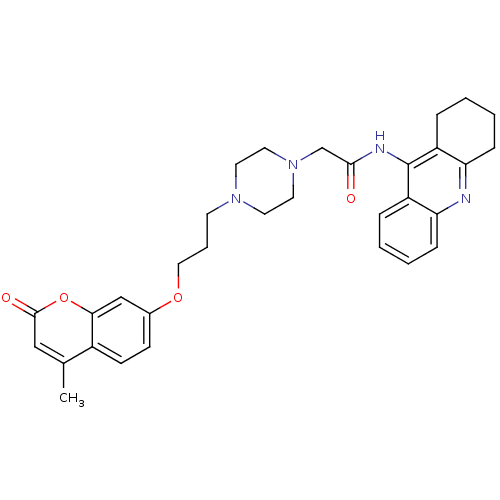 (CHEMBL2391487) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435955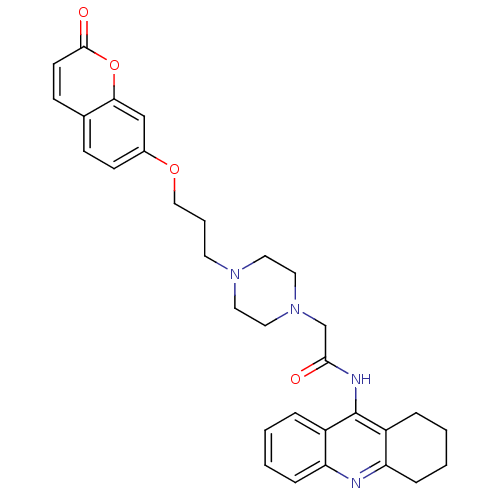 (CHEMBL2391654) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435961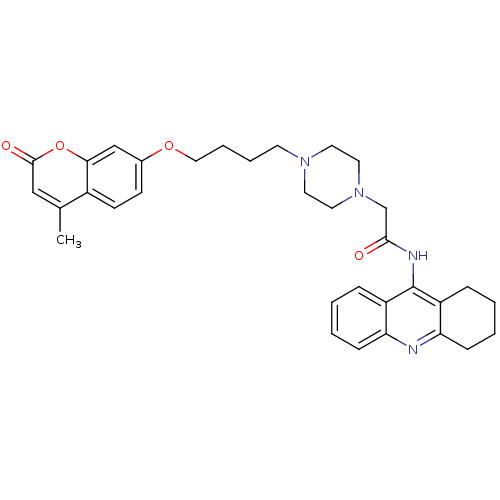 (CHEMBL2391488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435954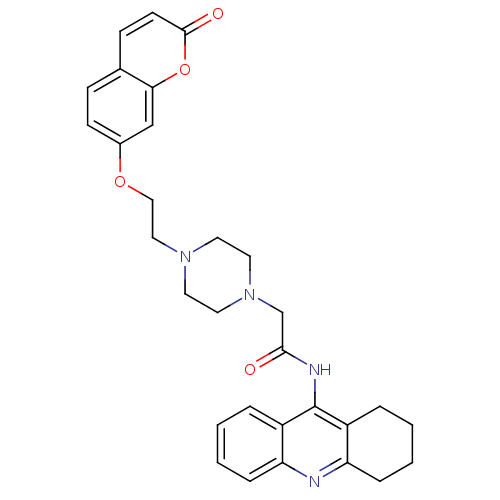 (CHEMBL2391653) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435942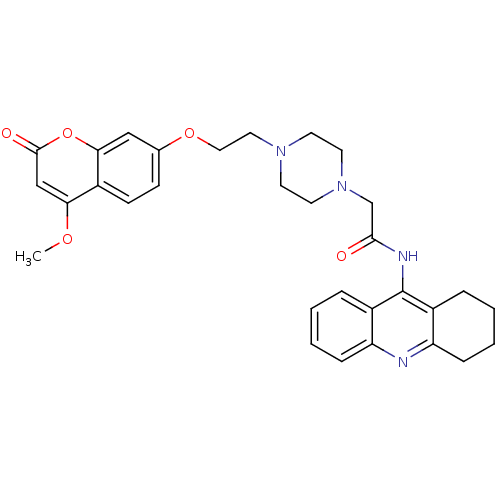 (CHEMBL2391491) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435957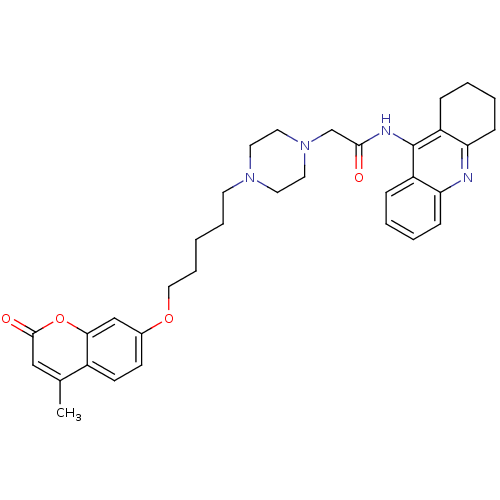 (CHEMBL2391489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435946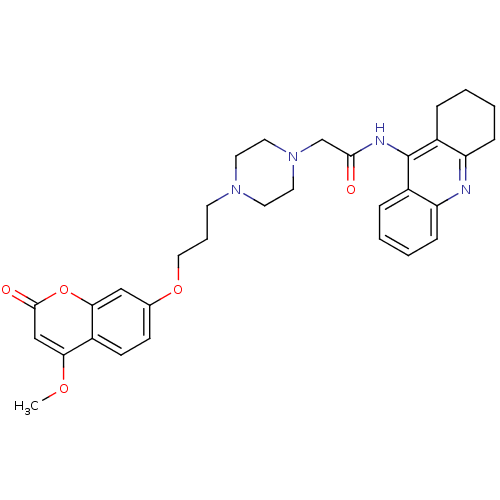 (CHEMBL2391492) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435959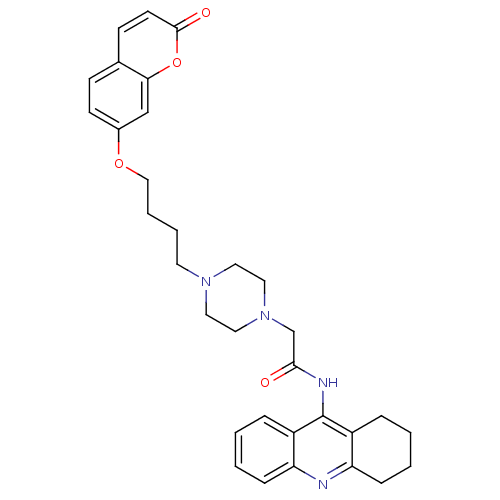 (CHEMBL2391483) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435961 (CHEMBL2391488) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435952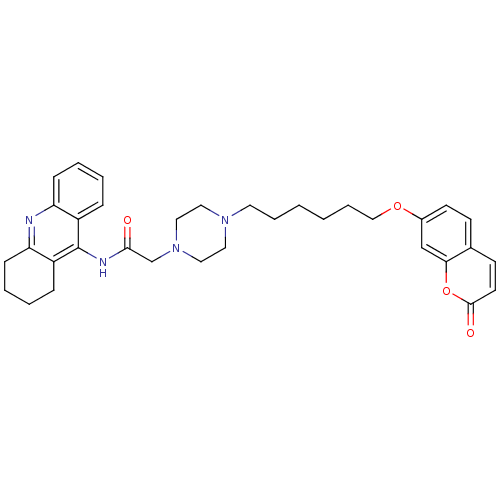 (CHEMBL2391485) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435958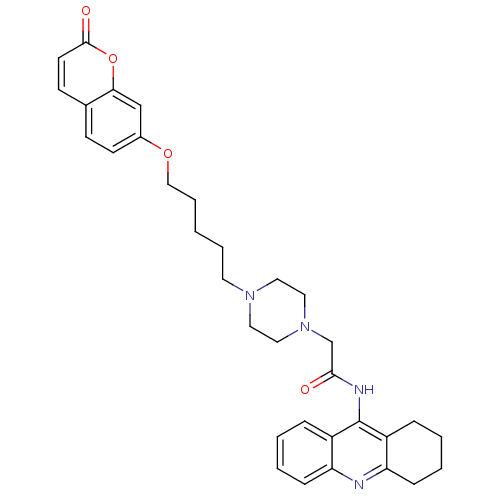 (CHEMBL2391484) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435960 (CHEMBL2391486) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435944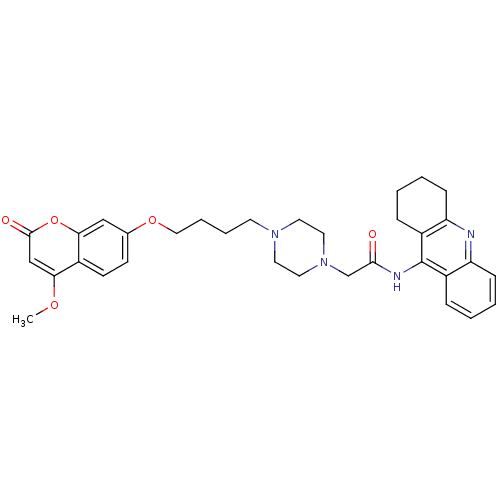 (CHEMBL2391475) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435959 (CHEMBL2391483) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435958 (CHEMBL2391484) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435957 (CHEMBL2391489) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 333 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435951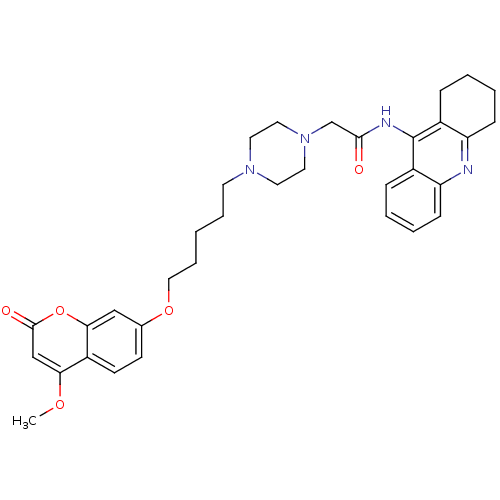 (CHEMBL2391476) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435950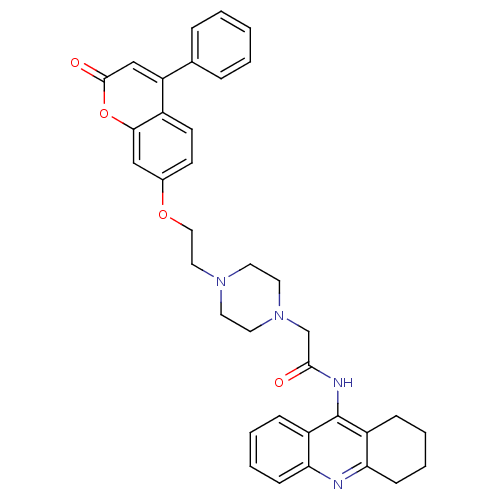 (CHEMBL2391478) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435956 (CHEMBL2391490) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435955 (CHEMBL2391654) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435954 (CHEMBL2391653) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435953 (CHEMBL2391487) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435952 (CHEMBL2391485) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435951 (CHEMBL2391476) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435945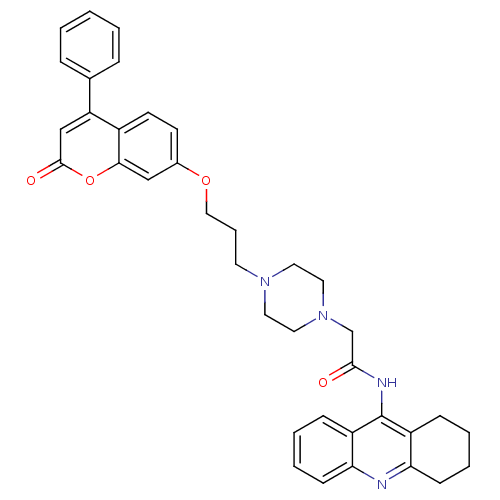 (CHEMBL2391479) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 887 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435948 (CHEMBL2391477) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 993 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435947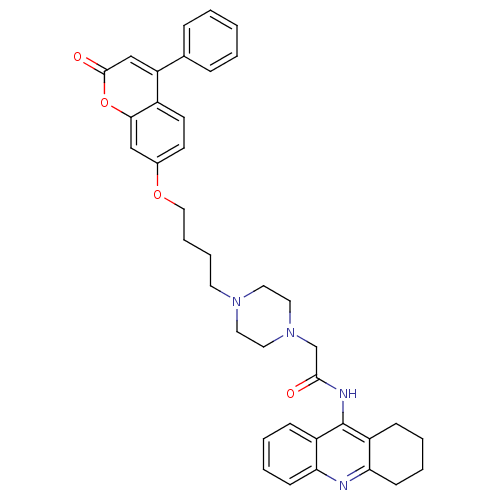 (CHEMBL2391480) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435949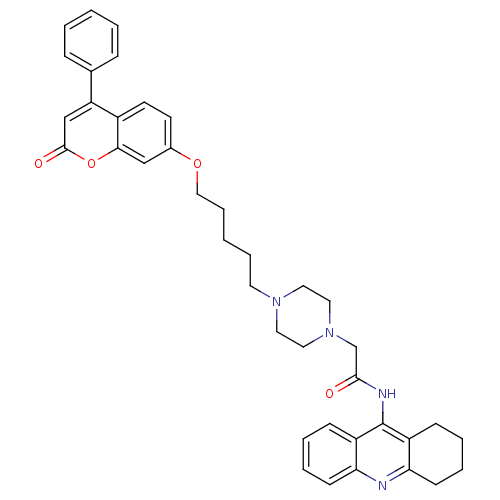 (CHEMBL2391481) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435950 (CHEMBL2391478) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435949 (CHEMBL2391481) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435948 (CHEMBL2391477) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435947 (CHEMBL2391480) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435946 (CHEMBL2391492) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435943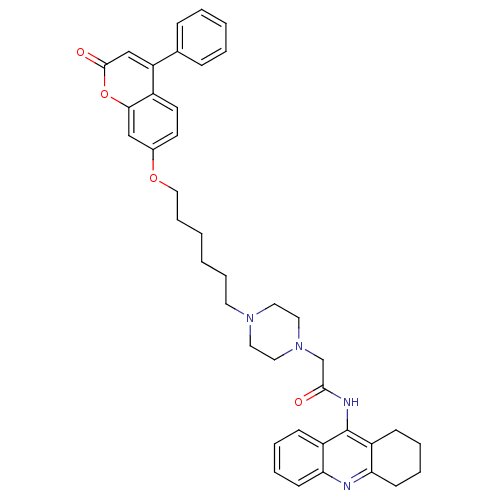 (CHEMBL2391482) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435945 (CHEMBL2391479) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435944 (CHEMBL2391475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435943 (CHEMBL2391482) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435942 (CHEMBL2391491) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435941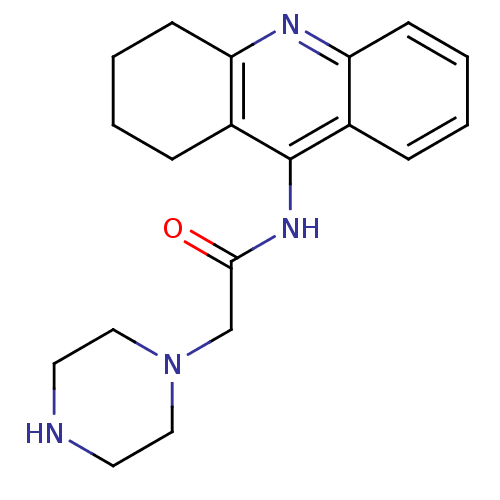 (CHEMBL2391652) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50174558 (7-hydroxy-2H-1-benzopyran-2-one | 7-hydroxy-2H-chr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435941 (CHEMBL2391652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50022178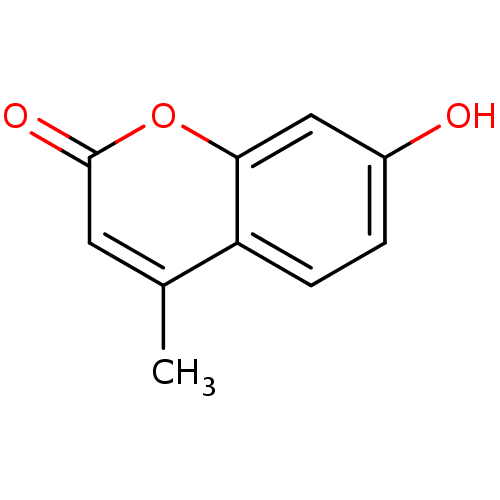 (4-Methyl-7-hydroxycoumarin | 4-methylumbelliferone...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50435940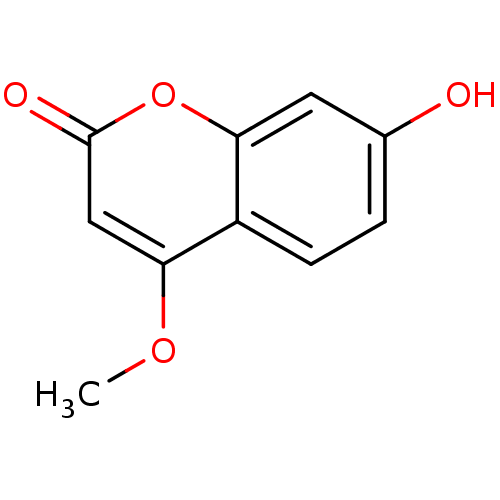 (CHEMBL2391651) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50104891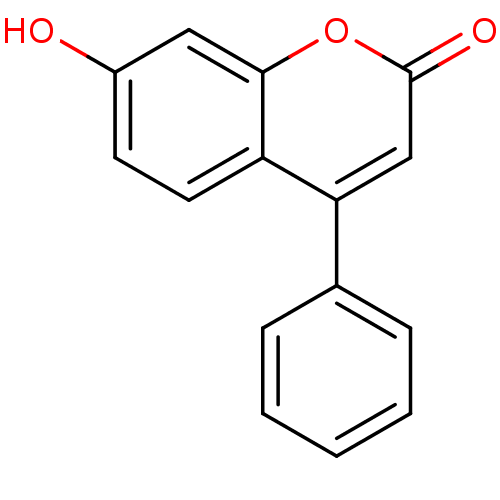 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to 180... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50174558 (7-hydroxy-2H-1-benzopyran-2-one | 7-hydroxy-2H-chr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50022178 (4-Methyl-7-hydroxycoumarin | 4-methylumbelliferone...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50104891 (7-Hydroxy-4-phenyl-chromen-2-one | 7-hydroxy-4-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50435940 (CHEMBL2391651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using S-butyrylthiocholine iodide as substrate preincubated for 6 mins prior to substrate addition measured at 60 to ... | Eur J Med Chem 64: 540-53 (2013) Article DOI: 10.1016/j.ejmech.2013.03.051 BindingDB Entry DOI: 10.7270/Q2H996KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||