

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061780 (CHEMBL3394226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061775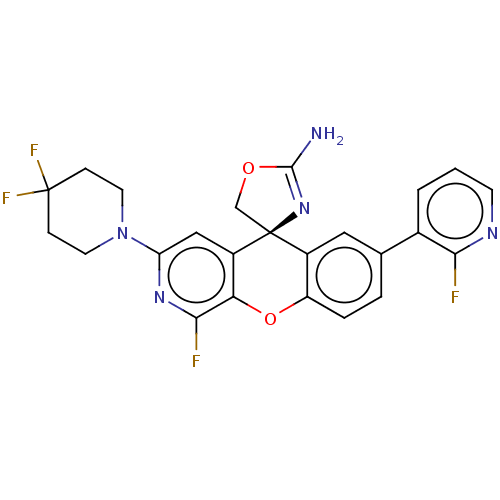 (CHEMBL3394225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061730 (CHEMBL3394216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061735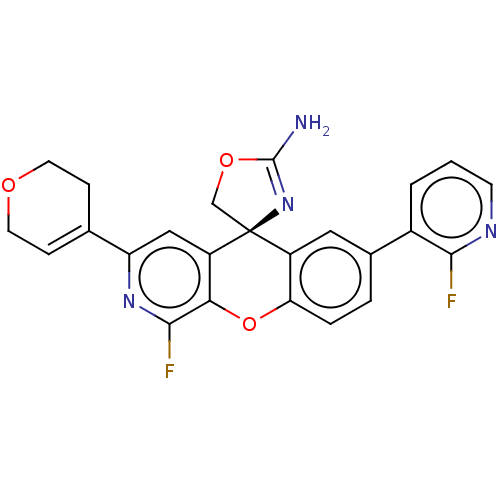 (CHEMBL3394221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061736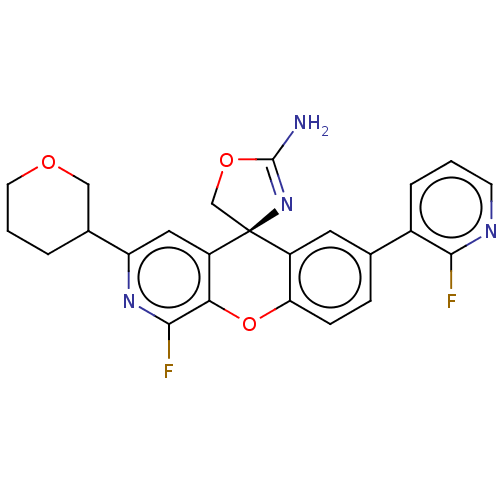 (CHEMBL3394222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061737 (CHEMBL3394223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061729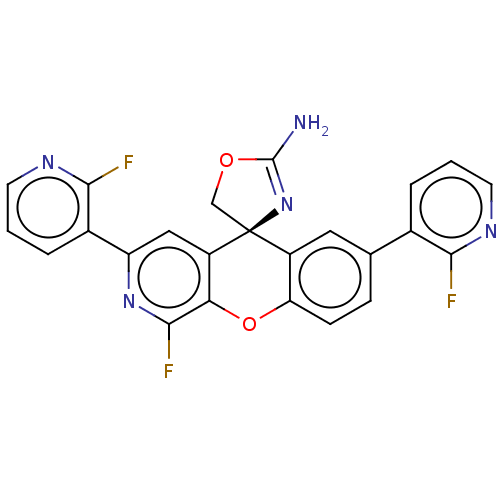 (CHEMBL3394215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061965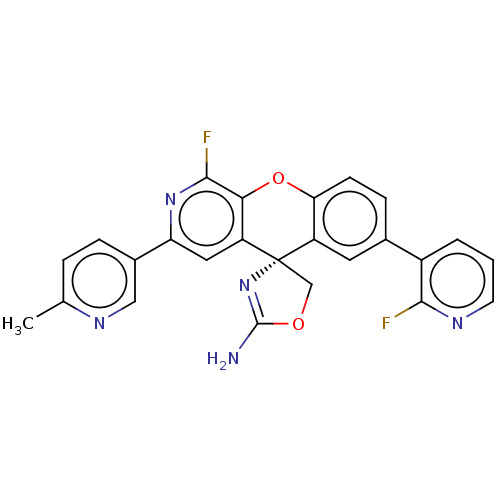 (CHEMBL3394213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061893 (CHEMBL3394212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061890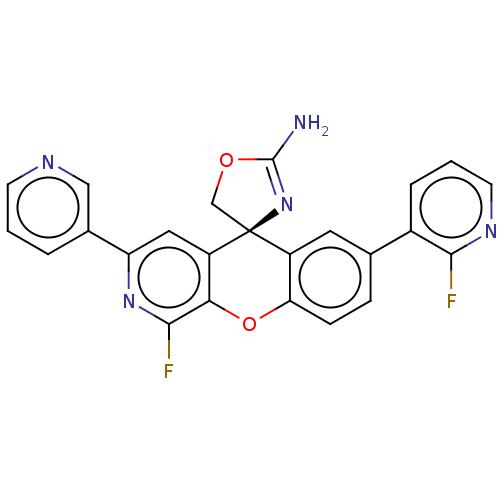 (CHEMBL3394211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061835 (CHEMBL3394228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061874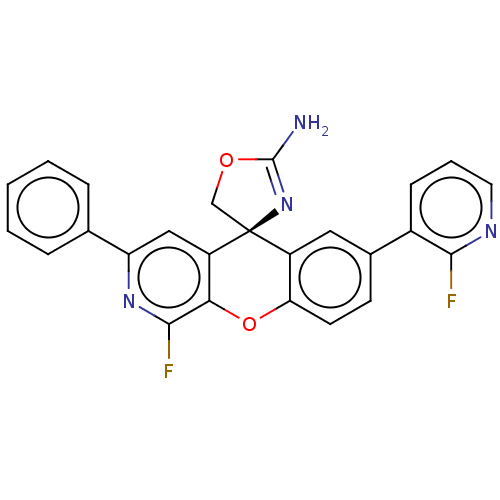 (CHEMBL3394210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061728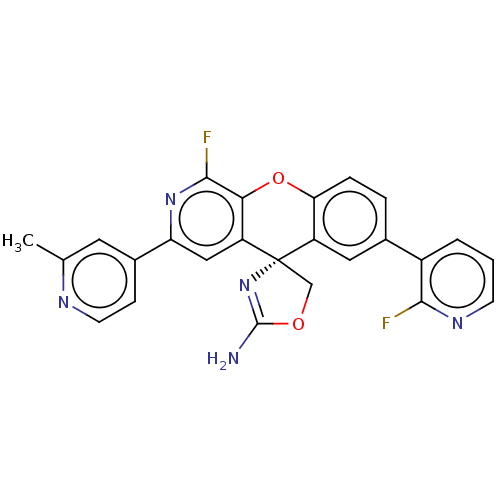 (CHEMBL3394214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061732 (CHEMBL3394218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061831 (CHEMBL3394227) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061733 (CHEMBL3394219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061738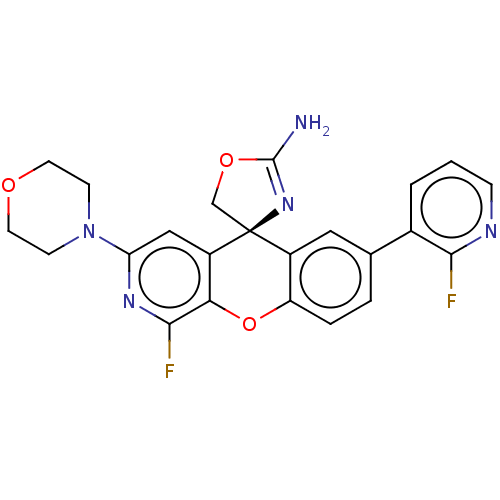 (CHEMBL3394224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061731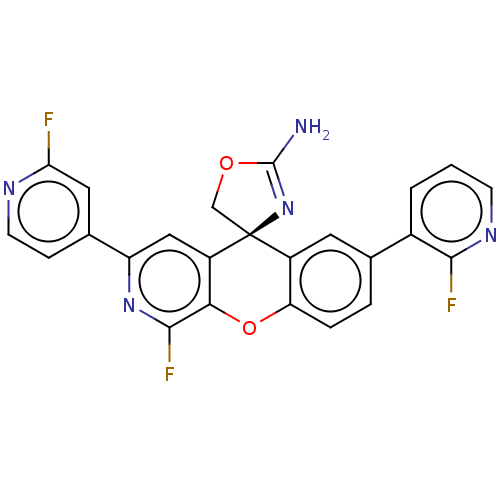 (CHEMBL3394217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50061734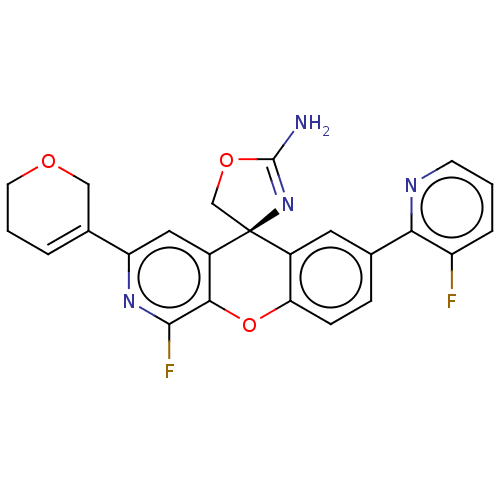 (CHEMBL3394220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061732 (CHEMBL3394218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061831 (CHEMBL3394227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061780 (CHEMBL3394226) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061890 (CHEMBL3394211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061735 (CHEMBL3394221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061775 (CHEMBL3394225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061734 (CHEMBL3394220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061730 (CHEMBL3394216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061738 (CHEMBL3394224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061835 (CHEMBL3394228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061733 (CHEMBL3394219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061893 (CHEMBL3394212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061731 (CHEMBL3394217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061965 (CHEMBL3394213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061737 (CHEMBL3394223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061874 (CHEMBL3394210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061728 (CHEMBL3394214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061736 (CHEMBL3394222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061729 (CHEMBL3394215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 pre-incubated with enzyme for 60 mins before fluorescent substrate addition FRET method | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061831 (CHEMBL3394227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061890 (CHEMBL3394211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061732 (CHEMBL3394218) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061735 (CHEMBL3394221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061734 (CHEMBL3394220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061780 (CHEMBL3394226) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061733 (CHEMBL3394219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061738 (CHEMBL3394224) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061965 (CHEMBL3394213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061775 (CHEMBL3394225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061893 (CHEMBL3394212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061731 (CHEMBL3394217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061874 (CHEMBL3394210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061728 (CHEMBL3394214) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061730 (CHEMBL3394216) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061737 (CHEMBL3394223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061835 (CHEMBL3394228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061729 (CHEMBL3394215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50061736 (CHEMBL3394222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells stably expressing APPSW assessed as reduction in Abeta40 level incubated overnight at 0.0005 to 10 uM by ELISA me... | ACS Med Chem Lett 6: 210-5 (2015) Article DOI: 10.1021/ml500458t BindingDB Entry DOI: 10.7270/Q2CN75JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||