

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046316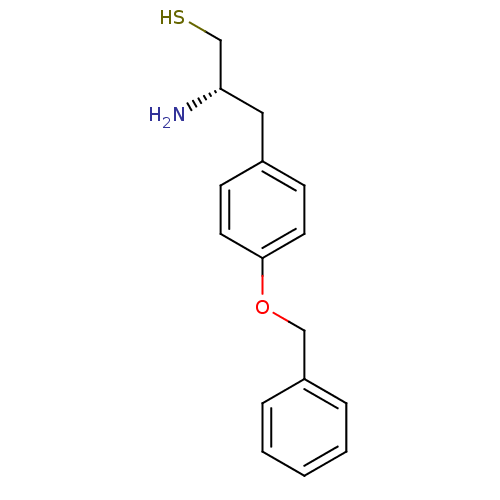 (2-Amino-3-(4-benzyloxy-phenyl)-propane-1-thiol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046314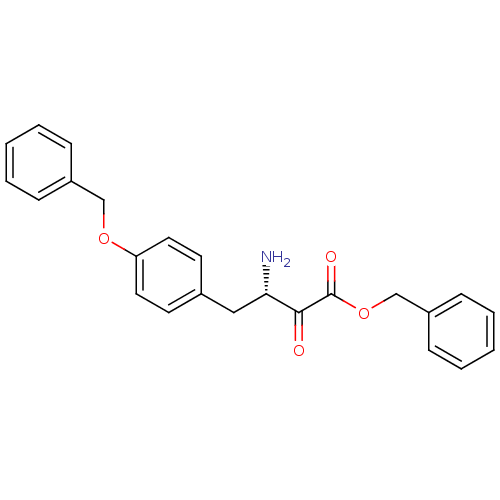 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50046316 (2-Amino-3-(4-benzyloxy-phenyl)-propane-1-thiol | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant was determined against epoxide hydrolase | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50046314 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition constant was determined against epoxide hydrolase | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046327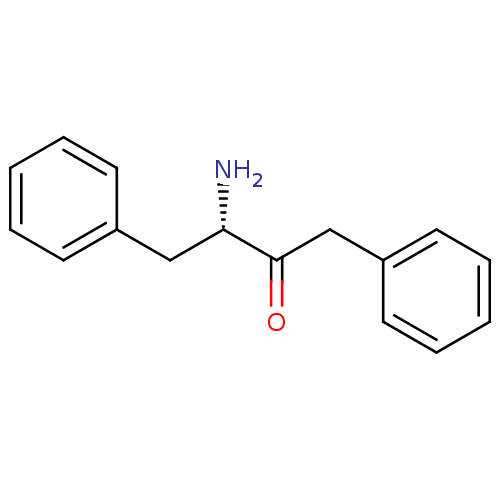 (3-Amino-1,4-diphenyl-butan-2-one | CHEMBL70658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046331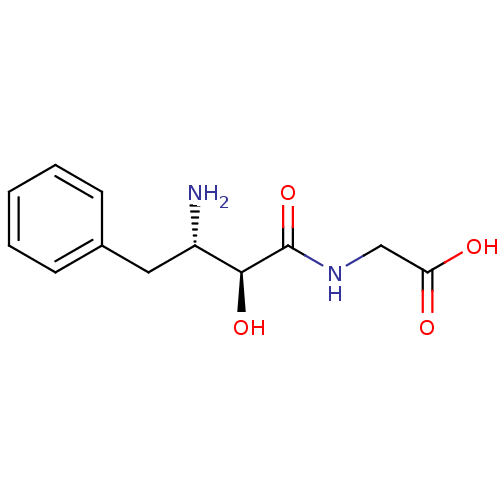 (((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyrylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50279808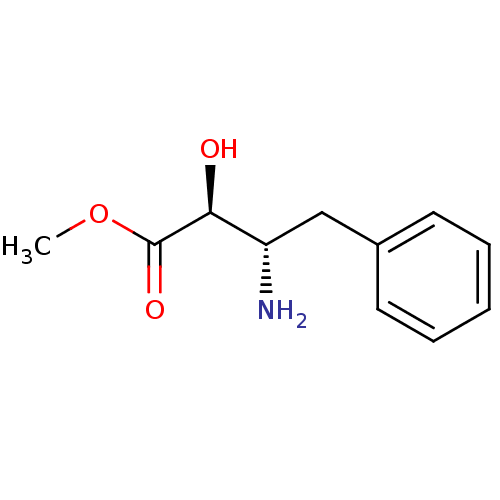 ((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyric acid me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Ability to inhibit amidase activity of LTA4 hydrolase (1.4 ug) purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046326 (2-(3-Amino-2-oxo-4-phenyl-butyrylamino)-4-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50046316 (2-Amino-3-(4-benzyloxy-phenyl)-propane-1-thiol | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against aminopeptidase M | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046329 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046321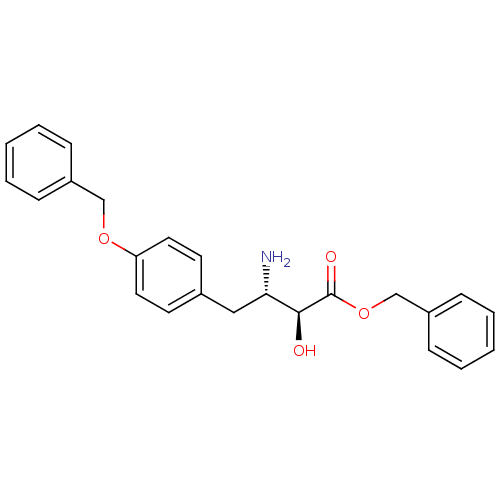 (3-Amino-4-(4-benzyloxy-phenyl)-2-hydroxy-butyric a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046319 (3-[2-Amino-3-(4-benzyloxy-phenyl)-propionyl]-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046315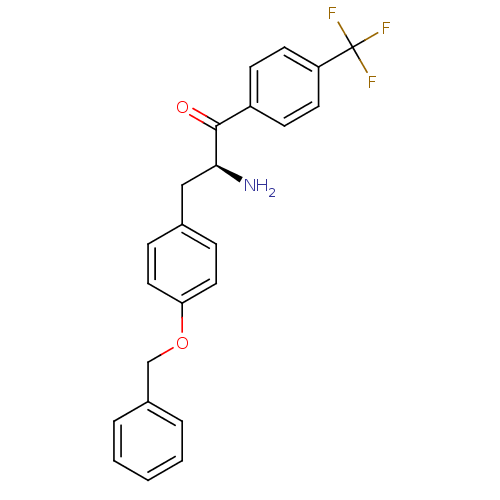 (2-Amino-3-(4-benzyloxy-phenyl)-1-(4-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046325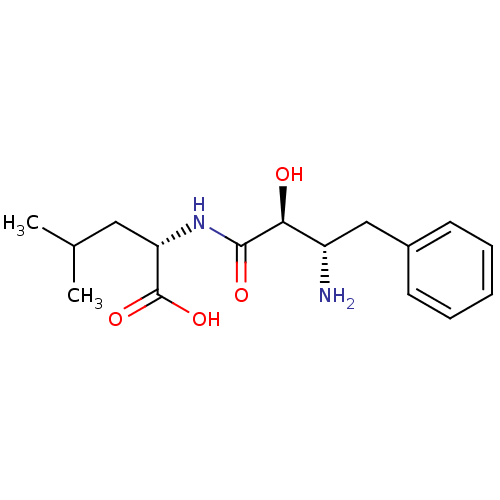 ((S)-2-((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyryla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046340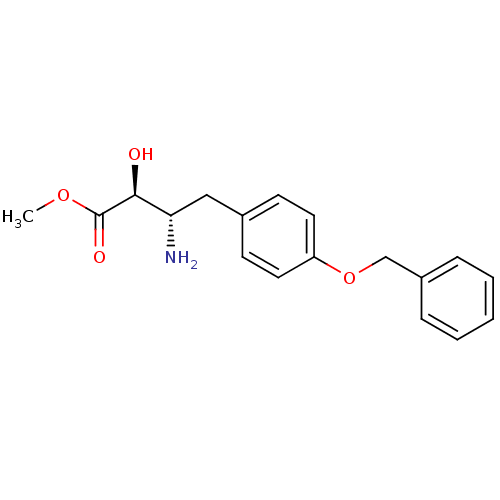 (3-Amino-4-(4-benzyloxy-phenyl)-2-hydroxy-butyric a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046335 (6-Amino-5-oxo-7-phenyl-heptanoic acid | CHEMBL3077...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046320 (3-Amino-2-hydroxy-4-phenyl-butyric acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50046329 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against cytosolic leucine aminopeptidase | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50046316 (2-Amino-3-(4-benzyloxy-phenyl)-propane-1-thiol | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against cytosolic leucine aminopeptidase | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046337 (3-((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyrylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50046329 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against aminopeptidase M | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046330 (3-Amino-2-oxo-4-phenyl-butyric acid methyl ester |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50046321 (3-Amino-4-(4-benzyloxy-phenyl)-2-hydroxy-butyric a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against cytosolic leucine aminopeptidase | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046318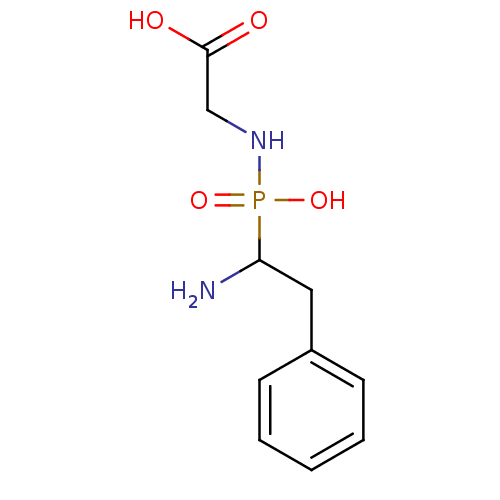 ((2-Amino-3-phenyl-ethylphosphonylamino)-acetic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046333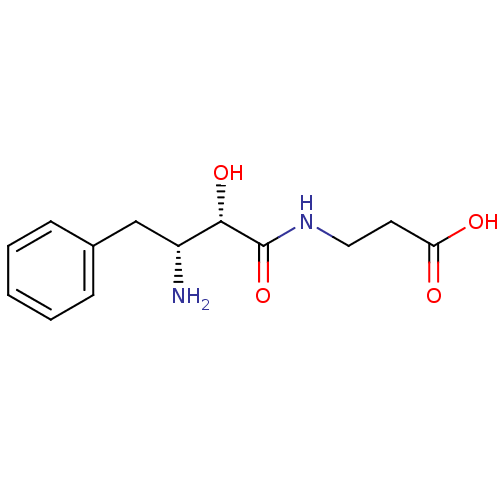 (3-((2S,3R)-3-Amino-2-hydroxy-4-phenyl-butyrylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50046321 (3-Amino-4-(4-benzyloxy-phenyl)-2-hydroxy-butyric a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against aminopeptidase M | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046334 (2-(3-Amino-2-hydroxy-4-phenyl-thiobutyrylamino)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50046314 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against cytosolic leucine aminopeptidase | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50022747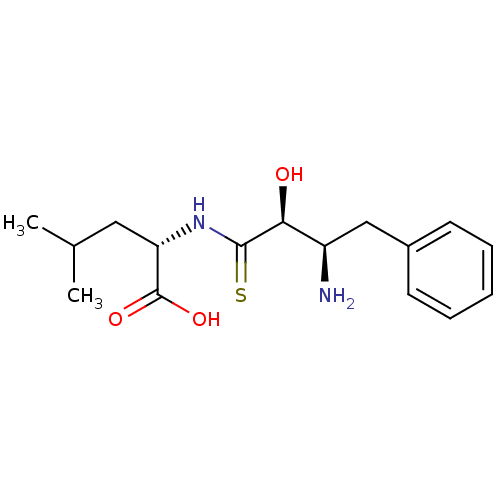 (2-(3-Amino-2-hydroxy-4-phenyl-thiobutyrylamino)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046328 (3-Amino-N-benzyl-2-hydroxy-4-phenyl-butyramide | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50046314 (3-Amino-4-(4-benzyloxy-phenyl)-2-oxo-butyric acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against aminopeptidase M | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046324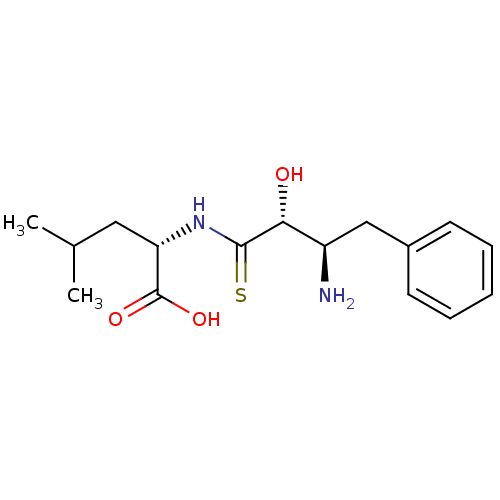 (2-(3-Amino-2-hydroxy-4-phenyl-thiobutyrylamino)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046313 (3-Amino-1,4-diphenyl-butan-2-ol | CHEMBL69297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046323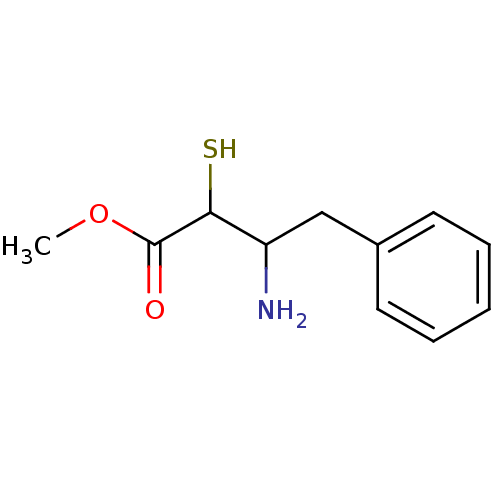 (3-Amino-2-mercapto-4-phenyl-butyric acid methyl es...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046317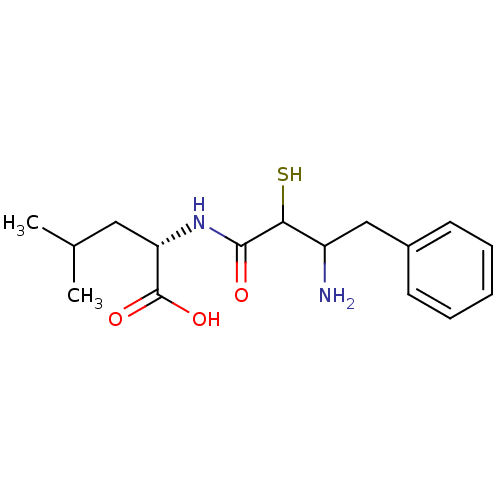 (2-(3-Amino-2-mercapto-4-phenyl-butyrylamino)-4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046322 ((2R,3S)-3-Amino-2-hydroxy-4-phenyl-butyric acid me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046339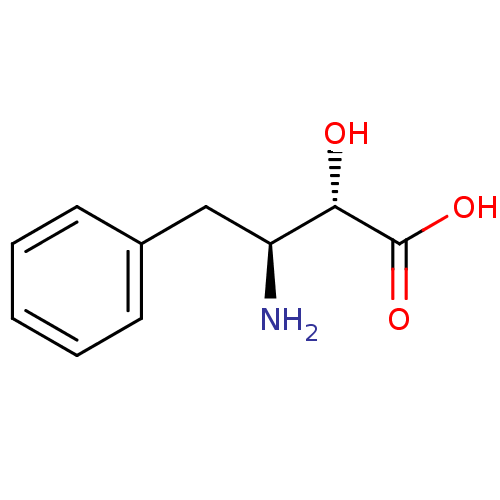 ((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyric acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046338 (3,3-Difluoro-4-(5-methyl-1H-imidazol-4-yl)-4-oxo-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50046332 ((E)-3,3-Difluoro-4-oxo-6-phenyl-hex-5-enoic acid e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes | J Med Chem 36: 211-20 (1993) BindingDB Entry DOI: 10.7270/Q2T152P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||