 Found 68 hits of Enzyme Inhibition Constant Data
Found 68 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055417

(CHEMBL407586 | Thielocin A1)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)C3=C(C)[C@@](C)(O)C4(O)Oc5c(C)c(OC)c(C(=O)Oc6c(C)c(C)c(C(O)=O)c(OC)c6C)c(C)c5C[C@@]4(C)C3=O)c(C)c2OC)c(C)c(C)c1C(O)=O |c:18| Show InChI InChI=1S/C54H60O18/c1-20-23(4)38(27(8)42(65-15)33(20)47(56)57)69-49(60)35-22(3)25(6)40(29(10)44(35)67-17)71-51(62)37-31(12)53(14,63)54(64)52(13,46(37)55)19-32-26(7)36(45(68-18)30(11)41(32)72-54)50(61)70-39-24(5)21(2)34(48(58)59)43(66-16)28(39)9/h63-64H,19H2,1-18H3,(H,56,57)(H,58,59)/t52-,53+,54?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055413

(CHEMBL384002 | Thielocin B3)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)cc(O)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)Oc6c(C)c(C)c(C(O)=O)c(OC)c6C)c(OC)c5C)c(O)c(C)c4O)c3O)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C62H66O20/c1-22-20-39(63)38(48(66)40(22)59(71)79-51-29(8)25(4)44(55(77-18)35(51)14)61(73)81-49-27(6)23(2)42(57(67)68)53(75-16)33(49)12)21-37-31(10)41(47(65)32(11)46(37)64)60(72)80-52-30(9)26(5)45(56(78-19)36(52)15)62(74)82-50-28(7)24(3)43(58(69)70)54(76-17)34(50)13/h20,63-66H,21H2,1-19H3,(H,67,68)(H,69,70) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055429
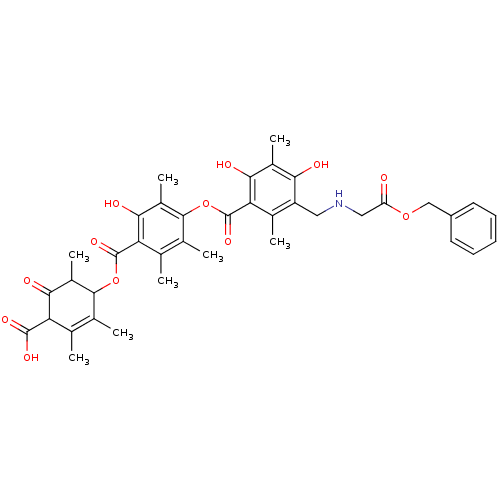
(4-[[[4-[[[5-[[[[(Benzyloxycarbonyl)methyl]amino]me...)Show SMILES CC1C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(CNCC(=O)OCc4ccccc4)c(O)c(C)c3O)c(C)c2O)C(C)=C(C)C(C(O)=O)C1=O |t:46| Show InChI InChI=1S/C39H43NO12/c1-17-19(3)35(23(7)33(44)28(17)37(46)47)51-38(48)29-18(2)20(4)36(24(8)34(29)45)52-39(49)30-21(5)26(31(42)22(6)32(30)43)14-40-15-27(41)50-16-25-12-10-9-11-13-25/h9-13,23,28,35,40,42-43,45H,14-16H2,1-8H3,(H,46,47) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055422

(3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1cc(OC)c(cc1Oc1cc(C)c(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C62H66O20/c1-25-21-38(22-40(48(25)73-16)60(68)80-50-33(9)29(5)47(56(77-20)37(50)13)62(70)82-52-31(7)27(3)45(58(65)66)54(75-18)35(52)11)78-43-23-39(41(71-14)24-42(43)72-15)59(67)79-49-32(8)28(4)46(55(76-19)36(49)12)61(69)81-51-30(6)26(2)44(57(63)64)53(74-17)34(51)10/h21-24H,1-20H3,(H,63,64)(H,65,66) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055424
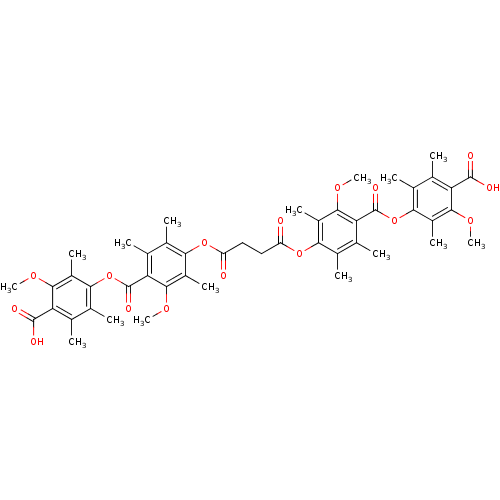
(CHEMBL146849 | Succinic acid bis-[4-(4-carboxy-3-m...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)CCC(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C48H54O16/c1-19-23(5)39(29(11)41(57-13)33(19)45(51)52)63-47(55)35-21(3)25(7)37(27(9)43(35)59-15)61-31(49)17-18-32(50)62-38-26(8)22(4)36(44(60-16)28(38)10)48(56)64-40-24(6)20(2)34(46(53)54)42(58-14)30(40)12/h17-18H2,1-16H3,(H,51,52)(H,53,54) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055431
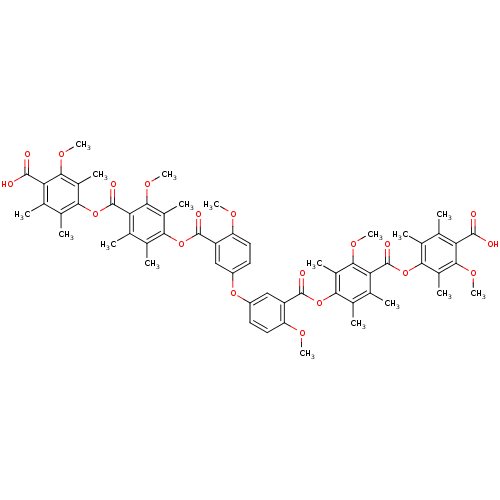
(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Oc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O19/c1-25-29(5)49(33(9)51(71-15)43(25)55(61)62)78-59(67)45-27(3)31(7)47(35(11)53(45)73-17)76-57(65)39-23-37(19-21-41(39)69-13)75-38-20-22-42(70-14)40(24-38)58(66)77-48-32(8)28(4)46(54(74-18)36(48)12)60(68)79-50-30(6)26(2)44(56(63)64)52(72-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055433

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O18S/c1-25-29(5)49(33(9)51(71-15)43(25)55(61)62)77-59(67)45-27(3)31(7)47(35(11)53(45)73-17)75-57(65)39-23-37(19-21-41(39)69-13)79-38-20-22-42(70-14)40(24-38)58(66)76-48-32(8)28(4)46(54(74-18)36(48)12)60(68)78-50-30(6)26(2)44(56(63)64)52(72-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055420
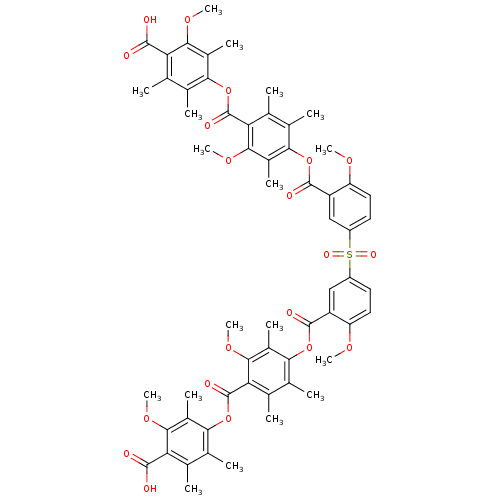
(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)S(=O)(=O)c1ccc(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O20S/c1-25-29(5)49(33(9)51(73-15)43(25)55(61)62)79-59(67)45-27(3)31(7)47(35(11)53(45)75-17)77-57(65)39-23-37(19-21-41(39)71-13)81(69,70)38-20-22-42(72-14)40(24-38)58(66)78-48-32(8)28(4)46(54(76-18)36(48)12)60(68)80-50-30(6)26(2)44(56(63)64)52(74-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055420

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)S(=O)(=O)c1ccc(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O20S/c1-25-29(5)49(33(9)51(73-15)43(25)55(61)62)79-59(67)45-27(3)31(7)47(35(11)53(45)75-17)77-57(65)39-23-37(19-21-41(39)71-13)81(69,70)38-20-22-42(72-14)40(24-38)58(66)78-48-32(8)28(4)46(54(76-18)36(48)12)60(68)80-50-30(6)26(2)44(56(63)64)52(74-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055413

(CHEMBL384002 | Thielocin B3)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)cc(O)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)Oc6c(C)c(C)c(C(O)=O)c(OC)c6C)c(OC)c5C)c(O)c(C)c4O)c3O)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C62H66O20/c1-22-20-39(63)38(48(66)40(22)59(71)79-51-29(8)25(4)44(55(77-18)35(51)14)61(73)81-49-27(6)23(2)42(57(67)68)53(75-16)33(49)12)21-37-31(10)41(47(65)32(11)46(37)64)60(72)80-52-30(9)26(5)45(56(78-19)36(52)15)62(74)82-50-28(7)24(3)43(58(69)70)54(76-17)34(50)13/h20,63-66H,21H2,1-19H3,(H,67,68)(H,69,70) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055431

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Oc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O19/c1-25-29(5)49(33(9)51(71-15)43(25)55(61)62)78-59(67)45-27(3)31(7)47(35(11)53(45)73-17)76-57(65)39-23-37(19-21-41(39)69-13)75-38-20-22-42(70-14)40(24-38)58(66)77-48-32(8)28(4)46(54(74-18)36(48)12)60(68)79-50-30(6)26(2)44(56(63)64)52(72-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055426

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)S(=O)c1ccc(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O19S/c1-25-29(5)49(33(9)51(72-15)43(25)55(61)62)78-59(67)45-27(3)31(7)47(35(11)53(45)74-17)76-57(65)39-23-37(19-21-41(39)70-13)80(69)38-20-22-42(71-14)40(24-38)58(66)77-48-32(8)28(4)46(54(75-18)36(48)12)60(68)79-50-30(6)26(2)44(56(63)64)52(73-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055419
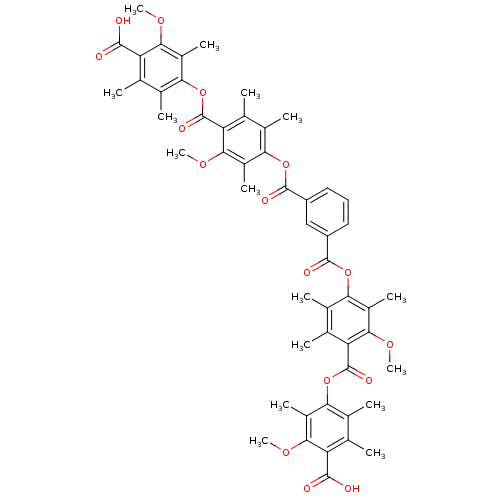
(CHEMBL358582 | Isophthalic acid bis-[4-(4-carboxy-...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3cccc(c3)C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C52H54O16/c1-21-25(5)41(29(9)43(61-13)35(21)47(53)54)67-51(59)37-23(3)27(7)39(31(11)45(37)63-15)65-49(57)33-18-17-19-34(20-33)50(58)66-40-28(8)24(4)38(46(64-16)32(40)12)52(60)68-42-26(6)22(2)36(48(55)56)44(62-14)30(42)10/h17-20H,1-16H3,(H,53,54)(H,55,56) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055430
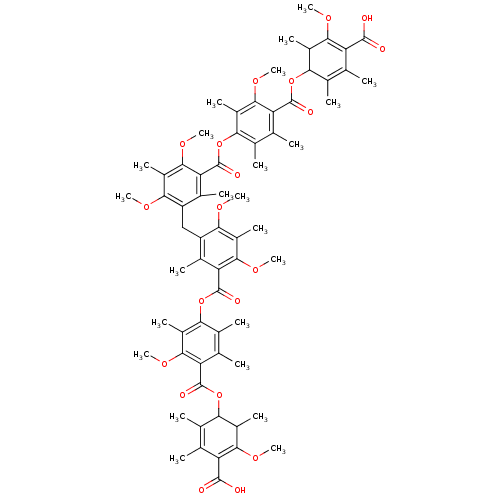
(Bis[5-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COC1=C(C(O)=O)C(C)=C(C)C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)OC6C(C)C(OC)=C(C(O)=O)C(C)=C6C)c(OC)c5C)c(OC)c(C)c4OC)c(OC)c(C)c3OC)c(C)c2OC)C1C |c:2,56,t:8,50| Show InChI InChI=1S/C67H80O20/c1-26-30(5)50(36(11)56(78-19)44(26)62(68)69)84-64(72)46-28(3)32(7)52(38(13)58(46)80-21)86-66(74)48-34(9)42(54(76-17)40(15)60(48)82-23)25-43-35(10)49(61(83-24)41(16)55(43)77-18)67(75)87-53-33(8)29(4)47(59(81-22)39(53)14)65(73)85-51-31(6)27(2)45(63(70)71)57(79-20)37(51)12/h36-37,50-51H,25H2,1-24H3,(H,68,69)(H,70,71) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055433

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O18S/c1-25-29(5)49(33(9)51(71-15)43(25)55(61)62)77-59(67)45-27(3)31(7)47(35(11)53(45)73-17)75-57(65)39-23-37(19-21-41(39)69-13)79-38-20-22-42(70-14)40(24-38)58(66)76-48-32(8)28(4)46(54(74-18)36(48)12)60(68)78-50-30(6)26(2)44(56(63)64)52(72-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055429

(4-[[[4-[[[5-[[[[(Benzyloxycarbonyl)methyl]amino]me...)Show SMILES CC1C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(CNCC(=O)OCc4ccccc4)c(O)c(C)c3O)c(C)c2O)C(C)=C(C)C(C(O)=O)C1=O |t:46| Show InChI InChI=1S/C39H43NO12/c1-17-19(3)35(23(7)33(44)28(17)37(46)47)51-38(48)29-18(2)20(4)36(24(8)34(29)45)52-39(49)30-21(5)26(31(42)22(6)32(30)43)14-40-15-27(41)50-16-25-12-10-9-11-13-25/h9-13,23,28,35,40,42-43,45H,14-16H2,1-8H3,(H,46,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055430

(Bis[5-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COC1=C(C(O)=O)C(C)=C(C)C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)OC6C(C)C(OC)=C(C(O)=O)C(C)=C6C)c(OC)c5C)c(OC)c(C)c4OC)c(OC)c(C)c3OC)c(C)c2OC)C1C |c:2,56,t:8,50| Show InChI InChI=1S/C67H80O20/c1-26-30(5)50(36(11)56(78-19)44(26)62(68)69)84-64(72)46-28(3)32(7)52(38(13)58(46)80-21)86-66(74)48-34(9)42(54(76-17)40(15)60(48)82-23)25-43-35(10)49(61(83-24)41(16)55(43)77-18)67(75)87-53-33(8)29(4)47(59(81-22)39(53)14)65(73)85-51-31(6)27(2)45(63(70)71)57(79-20)37(51)12/h36-37,50-51H,25H2,1-24H3,(H,68,69)(H,70,71) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055419

(CHEMBL358582 | Isophthalic acid bis-[4-(4-carboxy-...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3cccc(c3)C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C52H54O16/c1-21-25(5)41(29(9)43(61-13)35(21)47(53)54)67-51(59)37-23(3)27(7)39(31(11)45(37)63-15)65-49(57)33-18-17-19-34(20-33)50(58)66-40-28(8)24(4)38(46(64-16)32(40)12)52(60)68-42-26(6)22(2)36(48(55)56)44(62-14)30(42)10/h17-20H,1-16H3,(H,53,54)(H,55,56) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055422

(3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1cc(OC)c(cc1Oc1cc(C)c(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C62H66O20/c1-25-21-38(22-40(48(25)73-16)60(68)80-50-33(9)29(5)47(56(77-20)37(50)13)62(70)82-52-31(7)27(3)45(58(65)66)54(75-18)35(52)11)78-43-23-39(41(71-14)24-42(43)72-15)59(67)79-49-32(8)28(4)46(55(76-19)36(49)12)61(69)81-51-30(6)26(2)44(57(63)64)53(74-17)34(51)10/h21-24H,1-20H3,(H,63,64)(H,65,66) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055426

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)S(=O)c1ccc(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O19S/c1-25-29(5)49(33(9)51(72-15)43(25)55(61)62)78-59(67)45-27(3)31(7)47(35(11)53(45)74-17)76-57(65)39-23-37(19-21-41(39)70-13)80(69)38-20-22-42(71-14)40(24-38)58(66)77-48-32(8)28(4)46(54(75-18)36(48)12)60(68)79-50-30(6)26(2)44(56(63)64)52(73-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055414

((E)-But-2-enedioic acid bis-[4-(4-carboxy-3-methox...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)\C=C\C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C48H52O16/c1-19-23(5)39(29(11)41(57-13)33(19)45(51)52)63-47(55)35-21(3)25(7)37(27(9)43(35)59-15)61-31(49)17-18-32(50)62-38-26(8)22(4)36(44(60-16)28(38)10)48(56)64-40-24(6)20(2)34(46(53)54)42(58-14)30(40)12/h17-18H,1-16H3,(H,51,52)(H,53,54)/b18-17+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055427
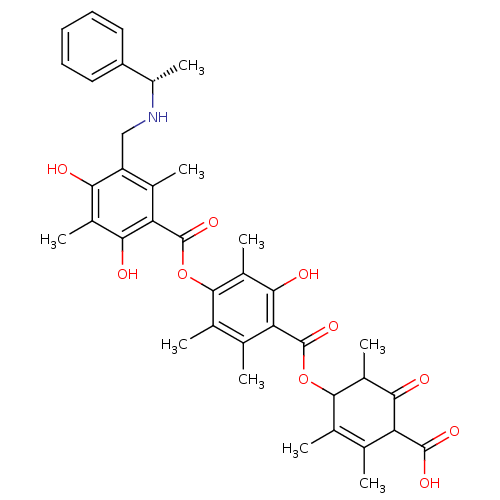
((S)-4-[[[4-[[[5[(sec-phenylamino)methyl]2,4-dihydr...)Show SMILES C[C@H](NCc1c(C)c(C(=O)Oc2c(C)c(C)c(C(=O)OC3C(C)C(=O)C(C(O)=O)C(C)=C3C)c(O)c2C)c(O)c(C)c1O)c1ccccc1 |c:30| Show InChI InChI=1S/C38H43NO10/c1-16-18(3)34(22(7)32(42)27(16)36(44)45)48-37(46)28-17(2)19(4)35(23(8)33(28)43)49-38(47)29-20(5)26(30(40)21(6)31(29)41)15-39-24(9)25-13-11-10-12-14-25/h10-14,22,24,27,34,39-41,43H,15H2,1-9H3,(H,44,45)/t22?,24-,27?,34?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055427

((S)-4-[[[4-[[[5[(sec-phenylamino)methyl]2,4-dihydr...)Show SMILES C[C@H](NCc1c(C)c(C(=O)Oc2c(C)c(C)c(C(=O)OC3C(C)C(=O)C(C(O)=O)C(C)=C3C)c(O)c2C)c(O)c(C)c1O)c1ccccc1 |c:30| Show InChI InChI=1S/C38H43NO10/c1-16-18(3)34(22(7)32(42)27(16)36(44)45)48-37(46)28-17(2)19(4)35(23(8)33(28)43)49-38(47)29-20(5)26(30(40)21(6)31(29)41)15-39-24(9)25-13-11-10-12-14-25/h10-14,22,24,27,34,39-41,43H,15H2,1-9H3,(H,44,45)/t22?,24-,27?,34?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055418

(4-Oxo-4H-pyran-2,6-dicarboxylic acid bis-[4-(4-car...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3cc(=O)cc(o3)C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C51H52O18/c1-19-23(5)40(27(9)42(61-13)34(19)46(53)54)68-50(59)36-21(3)25(7)38(29(11)44(36)63-15)66-48(57)32-17-31(52)18-33(65-32)49(58)67-39-26(8)22(4)37(45(64-16)30(39)12)51(60)69-41-24(6)20(2)35(47(55)56)43(62-14)28(41)10/h17-18H,1-16H3,(H,53,54)(H,55,56) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055424

(CHEMBL146849 | Succinic acid bis-[4-(4-carboxy-3-m...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)CCC(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C48H54O16/c1-19-23(5)39(29(11)41(57-13)33(19)45(51)52)63-47(55)35-21(3)25(7)37(27(9)43(35)59-15)61-31(49)17-18-32(50)62-38-26(8)22(4)36(44(60-16)28(38)10)48(56)64-40-24(6)20(2)34(46(53)54)42(58-14)30(40)12/h17-18H2,1-16H3,(H,51,52)(H,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055411

(Bis[5-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES CC1C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)OC6C(C)C(=O)C(C(O)=O)C(C)=C6C)c(O)c5C)c(O)c(C)c4O)c(O)c(C)c3O)c(C)c2O)C(C)=C(C)C(C(O)=O)C1=O |c:46,t:75| Show InChI InChI=1S/C59H64O20/c1-18-22(5)50(30(13)46(64)36(18)54(68)69)76-56(72)38-20(3)24(7)52(32(15)48(38)66)78-58(74)40-26(9)34(42(60)28(11)44(40)62)17-35-27(10)41(45(63)29(12)43(35)61)59(75)79-53-25(8)21(4)39(49(67)33(53)16)57(73)77-51-23(6)19(2)37(55(70)71)47(65)31(51)14/h30-31,36-37,50-51,60-63,66-67H,17H2,1-16H3,(H,68,69)(H,70,71) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50250399
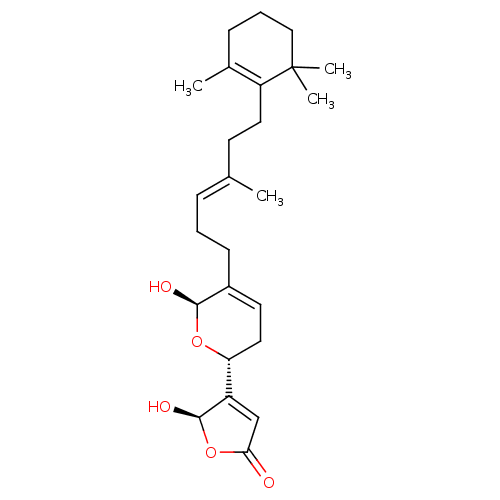
(5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...)Show SMILES C\C(CCC1=C(C)CCCC1(C)C)=C/CCC1=CC[C@@H](O[C@H]1O)C1=CC(=O)O[C@H]1O |r,c:4,t:17,25| Show InChI InChI=1S/C25H36O5/c1-16(10-12-20-17(2)8-6-14-25(20,3)4)7-5-9-18-11-13-21(29-23(18)27)19-15-22(26)30-24(19)28/h7,11,15,21,23-24,27-28H,5-6,8-10,12-14H2,1-4H3/b16-7+/t21-,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055412
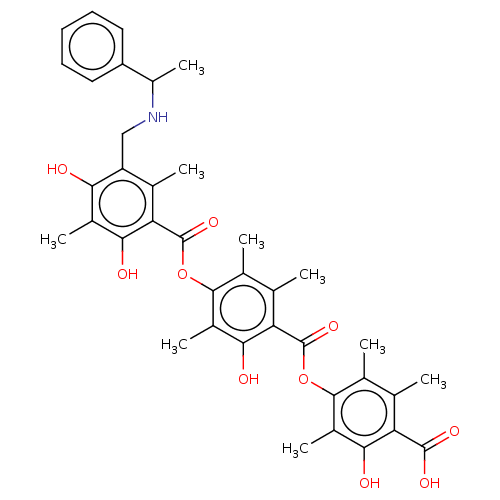
((R)-4-[[[4-[[[5[(sec-phenylamino)methyl]2,4-dihydr...)Show SMILES CC(NCc1c(C)c(C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(O)c3C)c(O)c2C)c(O)c(C)c1O)c1ccccc1 Show InChI InChI=1S/C38H41NO10/c1-16-18(3)34(22(7)32(42)27(16)36(44)45)48-37(46)28-17(2)19(4)35(23(8)33(28)43)49-38(47)29-20(5)26(30(40)21(6)31(29)41)15-39-24(9)25-13-11-10-12-14-25/h10-14,24,39-43H,15H2,1-9H3,(H,44,45) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055412

((R)-4-[[[4-[[[5[(sec-phenylamino)methyl]2,4-dihydr...)Show SMILES CC(NCc1c(C)c(C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(O)c3C)c(O)c2C)c(O)c(C)c1O)c1ccccc1 Show InChI InChI=1S/C38H41NO10/c1-16-18(3)34(22(7)32(42)27(16)36(44)45)48-37(46)28-17(2)19(4)35(23(8)33(28)43)49-38(47)29-20(5)26(30(40)21(6)31(29)41)15-39-24(9)25-13-11-10-12-14-25/h10-14,24,39-43H,15H2,1-9H3,(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055412

((R)-4-[[[4-[[[5[(sec-phenylamino)methyl]2,4-dihydr...)Show SMILES CC(NCc1c(C)c(C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(O)c3C)c(O)c2C)c(O)c(C)c1O)c1ccccc1 Show InChI InChI=1S/C38H41NO10/c1-16-18(3)34(22(7)32(42)27(16)36(44)45)48-37(46)28-17(2)19(4)35(23(8)33(28)43)49-38(47)29-20(5)26(30(40)21(6)31(29)41)15-39-24(9)25-13-11-10-12-14-25/h10-14,24,39-43H,15H2,1-9H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50250399

(5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...)Show SMILES C\C(CCC1=C(C)CCCC1(C)C)=C/CCC1=CC[C@@H](O[C@H]1O)C1=CC(=O)O[C@H]1O |r,c:4,t:17,25| Show InChI InChI=1S/C25H36O5/c1-16(10-12-20-17(2)8-6-14-25(20,3)4)7-5-9-18-11-13-21(29-23(18)27)19-15-22(26)30-24(19)28/h7,11,15,21,23-24,27-28H,5-6,8-10,12-14H2,1-4H3/b16-7+/t21-,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055428
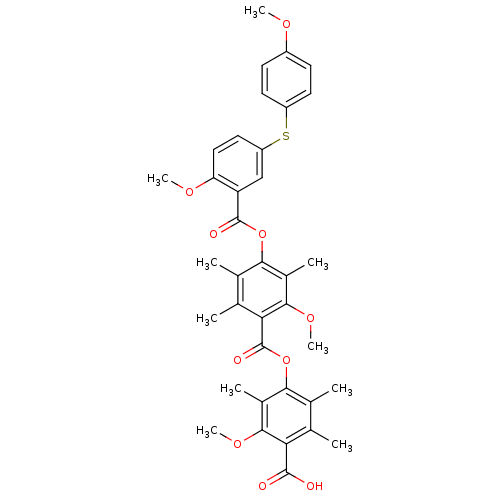
(3-carboxy-4-methoxyphenyl-3-[[4-[(4-carboxy-3-meth...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1 Show InChI InChI=1S/C37H38O10S/c1-18-20(3)32(22(5)33(44-9)29(18)35(38)39)47-37(41)30-19(2)21(4)31(23(6)34(30)45-10)46-36(40)27-17-26(15-16-28(27)43-8)48-25-13-11-24(42-7)12-14-25/h11-17H,1-10H3,(H,38,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055414

((E)-But-2-enedioic acid bis-[4-(4-carboxy-3-methox...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)\C=C\C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C48H52O16/c1-19-23(5)39(29(11)41(57-13)33(19)45(51)52)63-47(55)35-21(3)25(7)37(27(9)43(35)59-15)61-31(49)17-18-32(50)62-38-26(8)22(4)36(44(60-16)28(38)10)48(56)64-40-24(6)20(2)34(46(53)54)42(58-14)30(40)12/h17-18H,1-16H3,(H,51,52)(H,53,54)/b18-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055411

(Bis[5-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES CC1C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)OC6C(C)C(=O)C(C(O)=O)C(C)=C6C)c(O)c5C)c(O)c(C)c4O)c(O)c(C)c3O)c(C)c2O)C(C)=C(C)C(C(O)=O)C1=O |c:46,t:75| Show InChI InChI=1S/C59H64O20/c1-18-22(5)50(30(13)46(64)36(18)54(68)69)76-56(72)38-20(3)24(7)52(32(15)48(38)66)78-58(74)40-26(9)34(42(60)28(11)44(40)62)17-35-27(10)41(45(63)29(12)43(35)61)59(75)79-53-25(8)21(4)39(49(67)33(53)16)57(73)77-51-23(6)19(2)37(55(70)71)47(65)31(51)14/h30-31,36-37,50-51,60-63,66-67H,17H2,1-16H3,(H,68,69)(H,70,71) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055425
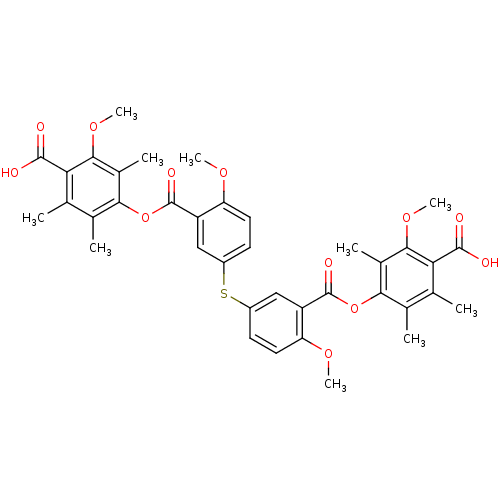
(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(O)=O)c(OC)c1C Show InChI InChI=1S/C38H38O12S/c1-17-19(3)31(21(5)33(47-9)29(17)35(39)40)49-37(43)25-15-23(11-13-27(25)45-7)51-24-12-14-28(46-8)26(16-24)38(44)50-32-20(4)18(2)30(36(41)42)34(48-10)22(32)6/h11-16H,1-10H3,(H,39,40)(H,41,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055415

(Bis[5-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1c(C)c(OC)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(C)c1Cc1c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c(C)c1OC Show InChI InChI=1S/C45H52O14/c1-18-20(3)34(24(7)38(54-13)30(18)42(46)47)58-44(50)32-22(5)28(36(52-11)26(9)40(32)56-15)17-29-23(6)33(41(57-16)27(10)37(29)53-12)45(51)59-35-21(4)19(2)31(43(48)49)39(55-14)25(35)8/h17H2,1-16H3,(H,46,47)(H,48,49) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055423
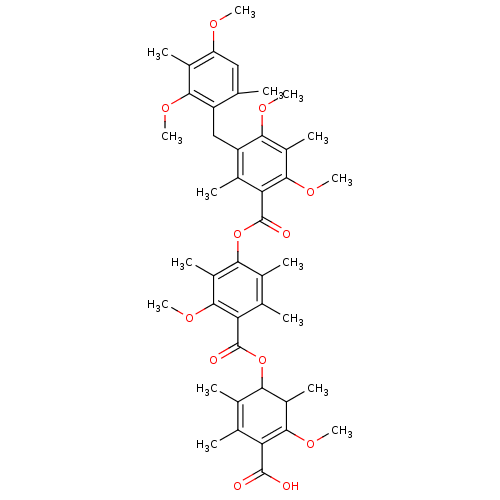
((5-carboxy-2,4-dimethoxy-3,6-dimethylphenyl)5-[[4-...)Show SMILES COC1=C(C(O)=O)C(C)=C(C)C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)cc(OC)c(C)c4OC)c(OC)c(C)c3OC)c(C)c2OC)C1C |c:2,t:8| Show InChI InChI=1S/C44H54O12/c1-19-17-31(49-11)25(7)37(50-12)29(19)18-30-24(6)34(41(54-16)28(10)38(30)51-13)44(48)56-36-23(5)21(3)33(40(53-15)27(36)9)43(47)55-35-22(4)20(2)32(42(45)46)39(52-14)26(35)8/h17,26,35H,18H2,1-16H3,(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055423

((5-carboxy-2,4-dimethoxy-3,6-dimethylphenyl)5-[[4-...)Show SMILES COC1=C(C(O)=O)C(C)=C(C)C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)cc(OC)c(C)c4OC)c(OC)c(C)c3OC)c(C)c2OC)C1C |c:2,t:8| Show InChI InChI=1S/C44H54O12/c1-19-17-31(49-11)25(7)37(50-12)29(19)18-30-24(6)34(41(54-16)28(10)38(30)51-13)44(48)56-36-23(5)21(3)33(40(53-15)27(36)9)43(47)55-35-22(4)20(2)32(42(45)46)39(52-14)26(35)8/h17,26,35H,18H2,1-16H3,(H,45,46) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055416

(Bis[3-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1ccc(Oc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(O)=O)c(OC)c1C Show InChI InChI=1S/C38H38O13/c1-17-19(3)31(21(5)33(47-9)29(17)35(39)40)50-37(43)25-15-23(11-13-27(25)45-7)49-24-12-14-28(46-8)26(16-24)38(44)51-32-20(4)18(2)30(36(41)42)34(48-10)22(32)6/h11-16H,1-10H3,(H,39,40)(H,41,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055428

(3-carboxy-4-methoxyphenyl-3-[[4-[(4-carboxy-3-meth...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1 Show InChI InChI=1S/C37H38O10S/c1-18-20(3)32(22(5)33(44-9)29(18)35(38)39)47-37(41)30-19(2)21(4)31(23(6)34(30)45-10)46-36(40)27-17-26(15-16-28(27)43-8)48-25-13-11-24(42-7)12-14-25/h11-17H,1-10H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50055415

(Bis[5-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1c(C)c(OC)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(C)c1Cc1c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c(C)c1OC Show InChI InChI=1S/C45H52O14/c1-18-20(3)34(24(7)38(54-13)30(18)42(46)47)58-44(50)32-22(5)28(36(52-11)26(9)40(32)56-15)17-29-23(6)33(41(57-16)27(10)37(29)53-12)45(51)59-35-21(4)19(2)31(43(48)49)39(55-14)25(35)8/h17H2,1-16H3,(H,46,47)(H,48,49) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055425

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(O)=O)c(OC)c1C Show InChI InChI=1S/C38H38O12S/c1-17-19(3)31(21(5)33(47-9)29(17)35(39)40)49-37(43)25-15-23(11-13-27(25)45-7)51-24-12-14-28(46-8)26(16-24)38(44)50-32-20(4)18(2)30(36(41)42)34(48-10)22(32)6/h11-16H,1-10H3,(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055418

(4-Oxo-4H-pyran-2,6-dicarboxylic acid bis-[4-(4-car...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3cc(=O)cc(o3)C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C51H52O18/c1-19-23(5)40(27(9)42(61-13)34(19)46(53)54)68-50(59)36-21(3)25(7)38(29(11)44(36)63-15)66-48(57)32-17-31(52)18-33(65-32)49(58)67-39-26(8)22(4)37(45(64-16)30(39)12)51(60)69-41-24(6)20(2)35(47(55)56)43(62-14)28(41)10/h17-18H,1-16H3,(H,53,54)(H,55,56) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055431

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Oc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O19/c1-25-29(5)49(33(9)51(71-15)43(25)55(61)62)78-59(67)45-27(3)31(7)47(35(11)53(45)73-17)76-57(65)39-23-37(19-21-41(39)69-13)75-38-20-22-42(70-14)40(24-38)58(66)77-48-32(8)28(4)46(54(74-18)36(48)12)60(68)79-50-30(6)26(2)44(56(63)64)52(72-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055422

(3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1cc(OC)c(cc1Oc1cc(C)c(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C62H66O20/c1-25-21-38(22-40(48(25)73-16)60(68)80-50-33(9)29(5)47(56(77-20)37(50)13)62(70)82-52-31(7)27(3)45(58(65)66)54(75-18)35(52)11)78-43-23-39(41(71-14)24-42(43)72-15)59(67)79-49-32(8)28(4)46(55(76-19)36(49)12)61(69)81-51-30(6)26(2)44(57(63)64)53(74-17)34(51)10/h21-24H,1-20H3,(H,63,64)(H,65,66) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055420

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)S(=O)(=O)c1ccc(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O20S/c1-25-29(5)49(33(9)51(73-15)43(25)55(61)62)79-59(67)45-27(3)31(7)47(35(11)53(45)75-17)77-57(65)39-23-37(19-21-41(39)71-13)81(69,70)38-20-22-42(72-14)40(24-38)58(66)78-48-32(8)28(4)46(54(76-18)36(48)12)60(68)80-50-30(6)26(2)44(56(63)64)52(74-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055429

(4-[[[4-[[[5-[[[[(Benzyloxycarbonyl)methyl]amino]me...)Show SMILES CC1C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(CNCC(=O)OCc4ccccc4)c(O)c(C)c3O)c(C)c2O)C(C)=C(C)C(C(O)=O)C1=O |t:46| Show InChI InChI=1S/C39H43NO12/c1-17-19(3)35(23(7)33(44)28(17)37(46)47)51-38(48)29-18(2)20(4)36(24(8)34(29)45)52-39(49)30-21(5)26(31(42)22(6)32(30)43)14-40-15-27(41)50-16-25-12-10-9-11-13-25/h9-13,23,28,35,40,42-43,45H,14-16H2,1-8H3,(H,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055426

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C)S(=O)c1ccc(OC)c(c1)C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O19S/c1-25-29(5)49(33(9)51(72-15)43(25)55(61)62)78-59(67)45-27(3)31(7)47(35(11)53(45)74-17)76-57(65)39-23-37(19-21-41(39)70-13)80(69)38-20-22-42(71-14)40(24-38)58(66)77-48-32(8)28(4)46(54(75-18)36(48)12)60(68)79-50-30(6)26(2)44(56(63)64)52(73-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055417

(CHEMBL407586 | Thielocin A1)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)C3=C(C)[C@@](C)(O)C4(O)Oc5c(C)c(OC)c(C(=O)Oc6c(C)c(C)c(C(O)=O)c(OC)c6C)c(C)c5C[C@@]4(C)C3=O)c(C)c2OC)c(C)c(C)c1C(O)=O |c:18| Show InChI InChI=1S/C54H60O18/c1-20-23(4)38(27(8)42(65-15)33(20)47(56)57)69-49(60)35-22(3)25(6)40(29(10)44(35)67-17)71-51(62)37-31(12)53(14,63)54(64)52(13,46(37)55)19-32-26(7)36(45(68-18)30(11)41(32)72-54)50(61)70-39-24(5)21(2)34(48(58)59)43(66-16)28(39)9/h63-64H,19H2,1-18H3,(H,56,57)(H,58,59)/t52-,53+,54?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055413

(CHEMBL384002 | Thielocin B3)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)cc(O)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)Oc6c(C)c(C)c(C(O)=O)c(OC)c6C)c(OC)c5C)c(O)c(C)c4O)c3O)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C62H66O20/c1-22-20-39(63)38(48(66)40(22)59(71)79-51-29(8)25(4)44(55(77-18)35(51)14)61(73)81-49-27(6)23(2)42(57(67)68)53(75-16)33(49)12)21-37-31(10)41(47(65)32(11)46(37)64)60(72)80-52-30(9)26(5)45(56(78-19)36(52)15)62(74)82-50-28(7)24(3)43(58(69)70)54(76-17)34(50)13/h20,63-66H,21H2,1-19H3,(H,67,68)(H,69,70) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055433

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C60H62O18S/c1-25-29(5)49(33(9)51(71-15)43(25)55(61)62)77-59(67)45-27(3)31(7)47(35(11)53(45)73-17)75-57(65)39-23-37(19-21-41(39)69-13)79-38-20-22-42(70-14)40(24-38)58(66)76-48-32(8)28(4)46(54(74-18)36(48)12)60(68)78-50-30(6)26(2)44(56(63)64)52(72-16)34(50)10/h19-24H,1-18H3,(H,61,62)(H,63,64) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055427

((S)-4-[[[4-[[[5[(sec-phenylamino)methyl]2,4-dihydr...)Show SMILES C[C@H](NCc1c(C)c(C(=O)Oc2c(C)c(C)c(C(=O)OC3C(C)C(=O)C(C(O)=O)C(C)=C3C)c(O)c2C)c(O)c(C)c1O)c1ccccc1 |c:30| Show InChI InChI=1S/C38H43NO10/c1-16-18(3)34(22(7)32(42)27(16)36(44)45)48-37(46)28-17(2)19(4)35(23(8)33(28)43)49-38(47)29-20(5)26(30(40)21(6)31(29)41)15-39-24(9)25-13-11-10-12-14-25/h10-14,22,24,27,34,39-41,43H,15H2,1-9H3,(H,44,45)/t22?,24-,27?,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055424

(CHEMBL146849 | Succinic acid bis-[4-(4-carboxy-3-m...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)CCC(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C48H54O16/c1-19-23(5)39(29(11)41(57-13)33(19)45(51)52)63-47(55)35-21(3)25(7)37(27(9)43(35)59-15)61-31(49)17-18-32(50)62-38-26(8)22(4)36(44(60-16)28(38)10)48(56)64-40-24(6)20(2)34(46(53)54)42(58-14)30(40)12/h17-18H2,1-16H3,(H,51,52)(H,53,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055416

(Bis[3-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1ccc(Oc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(O)=O)c(OC)c1C Show InChI InChI=1S/C38H38O13/c1-17-19(3)31(21(5)33(47-9)29(17)35(39)40)50-37(43)25-15-23(11-13-27(25)45-7)49-24-12-14-28(46-8)26(16-24)38(44)51-32-20(4)18(2)30(36(41)42)34(48-10)22(32)6/h11-16H,1-10H3,(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50015214

(6-chloro-2-methoxy-9-acridinyl(4-diethylamino-1-me...)Show SMILES CCN(CC)CCCC(C)Nc1c2ccc(Cl)cc2nc2ccc(OC)cc12 Show InChI InChI=1S/C23H30ClN3O/c1-5-27(6-2)13-7-8-16(3)25-23-19-11-9-17(24)14-22(19)26-21-12-10-18(28-4)15-20(21)23/h9-12,14-16H,5-8,13H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055415

(Bis[5-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1c(C)c(OC)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(C)c1Cc1c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c(C)c1OC Show InChI InChI=1S/C45H52O14/c1-18-20(3)34(24(7)38(54-13)30(18)42(46)47)58-44(50)32-22(5)28(36(52-11)26(9)40(32)56-15)17-29-23(6)33(41(57-16)27(10)37(29)53-12)45(51)59-35-21(4)19(2)31(43(48)49)39(55-14)25(35)8/h17H2,1-16H3,(H,46,47)(H,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055416

(Bis[3-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COc1ccc(Oc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(O)=O)c(OC)c1C Show InChI InChI=1S/C38H38O13/c1-17-19(3)31(21(5)33(47-9)29(17)35(39)40)50-37(43)25-15-23(11-13-27(25)45-7)49-24-12-14-28(46-8)26(16-24)38(44)51-32-20(4)18(2)30(36(41)42)34(48-10)22(32)6/h11-16H,1-10H3,(H,39,40)(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055430

(Bis[5-[(4-carboxy-3-methoxy-2,5,6-trimethylphenoxy...)Show SMILES COC1=C(C(O)=O)C(C)=C(C)C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)OC6C(C)C(OC)=C(C(O)=O)C(C)=C6C)c(OC)c5C)c(OC)c(C)c4OC)c(OC)c(C)c3OC)c(C)c2OC)C1C |c:2,56,t:8,50| Show InChI InChI=1S/C67H80O20/c1-26-30(5)50(36(11)56(78-19)44(26)62(68)69)84-64(72)46-28(3)32(7)52(38(13)58(46)80-21)86-66(74)48-34(9)42(54(76-17)40(15)60(48)82-23)25-43-35(10)49(61(83-24)41(16)55(43)77-18)67(75)87-53-33(8)29(4)47(59(81-22)39(53)14)65(73)85-51-31(6)27(2)45(63(70)71)57(79-20)37(51)12/h36-37,50-51H,25H2,1-24H3,(H,68,69)(H,70,71) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055414

((E)-But-2-enedioic acid bis-[4-(4-carboxy-3-methox...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)\C=C\C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C48H52O16/c1-19-23(5)39(29(11)41(57-13)33(19)45(51)52)63-47(55)35-21(3)25(7)37(27(9)43(35)59-15)61-31(49)17-18-32(50)62-38-26(8)22(4)36(44(60-16)28(38)10)48(56)64-40-24(6)20(2)34(46(53)54)42(58-14)30(40)12/h17-18H,1-16H3,(H,51,52)(H,53,54)/b18-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055428

(3-carboxy-4-methoxyphenyl-3-[[4-[(4-carboxy-3-meth...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(O)=O)c(OC)c3C)c(OC)c2C)cc1 Show InChI InChI=1S/C37H38O10S/c1-18-20(3)32(22(5)33(44-9)29(18)35(38)39)47-37(41)30-19(2)21(4)31(23(6)34(30)45-10)46-36(40)27-17-26(15-16-28(27)43-8)48-25-13-11-24(42-7)12-14-25/h11-17H,1-10H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055423

((5-carboxy-2,4-dimethoxy-3,6-dimethylphenyl)5-[[4-...)Show SMILES COC1=C(C(O)=O)C(C)=C(C)C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)cc(OC)c(C)c4OC)c(OC)c(C)c3OC)c(C)c2OC)C1C |c:2,t:8| Show InChI InChI=1S/C44H54O12/c1-19-17-31(49-11)25(7)37(50-12)29(19)18-30-24(6)34(41(54-16)28(10)38(30)51-13)44(48)56-36-23(5)21(3)33(40(53-15)27(36)9)43(47)55-35-22(4)20(2)32(42(45)46)39(52-14)26(35)8/h17,26,35H,18H2,1-16H3,(H,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055418

(4-Oxo-4H-pyran-2,6-dicarboxylic acid bis-[4-(4-car...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3cc(=O)cc(o3)C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C51H52O18/c1-19-23(5)40(27(9)42(61-13)34(19)46(53)54)68-50(59)36-21(3)25(7)38(29(11)44(36)63-15)66-48(57)32-17-31(52)18-33(65-32)49(58)67-39-26(8)22(4)37(45(64-16)30(39)12)51(60)69-41-24(6)20(2)35(47(55)56)43(62-14)28(41)10/h17-18H,1-16H3,(H,53,54)(H,55,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055425

(Bis[3-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(O)=O)c(OC)c1C Show InChI InChI=1S/C38H38O12S/c1-17-19(3)31(21(5)33(47-9)29(17)35(39)40)49-37(43)25-15-23(11-13-27(25)45-7)51-24-12-14-28(46-8)26(16-24)38(44)50-32-20(4)18(2)30(36(41)42)34(48-10)22(32)6/h11-16H,1-10H3,(H,39,40)(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055421
(Bis[3-[[4-[[4-[[[(pivaloyloxy)methyl]carbonyl]-3-m...)Show SMILES COc1ccc(Sc2ccc(OC)c(c2)C(=O)Oc2c(C)c(C)c(C(=O)Oc3c(C)c(C)c(C(=O)OCOC(=O)C(C)(C)C)c(OC)c3C)c(OC)c2C)cc1C(=O)Oc1c(C)c(C)c(C(=O)Oc2c(C)c(C)c(C(O)=O)c(OC)c2C)c(OC)c1C Show InChI InChI=1S/C66H72O20S/c1-29-33(5)53(37(9)55(77-18)47(29)59(67)68)85-63(72)49-31(3)35(7)51(38(10)57(49)79-20)83-60(69)43-26-41(22-24-45(43)75-16)87-42-23-25-46(76-17)44(27-42)61(70)84-52-36(8)32(4)50(58(80-21)39(52)11)64(73)86-54-34(6)30(2)48(56(78-19)40(54)12)62(71)81-28-82-65(74)66(13,14)15/h22-27H,28H2,1-21H3,(H,67,68) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055411

(Bis[5-[[4-[(4-carboxy-3-methoxy-2,5,6-trimethylphe...)Show SMILES CC1C(OC(=O)c2c(C)c(C)c(OC(=O)c3c(C)c(Cc4c(C)c(C(=O)Oc5c(C)c(C)c(C(=O)OC6C(C)C(=O)C(C(O)=O)C(C)=C6C)c(O)c5C)c(O)c(C)c4O)c(O)c(C)c3O)c(C)c2O)C(C)=C(C)C(C(O)=O)C1=O |c:46,t:75| Show InChI InChI=1S/C59H64O20/c1-18-22(5)50(30(13)46(64)36(18)54(68)69)76-56(72)38-20(3)24(7)52(32(15)48(38)66)78-58(74)40-26(9)34(42(60)28(11)44(40)62)17-35-27(10)41(45(63)29(12)43(35)61)59(75)79-53-25(8)21(4)39(49(67)33(53)16)57(73)77-51-23(6)19(2)37(55(70)71)47(65)31(51)14/h30-31,36-37,50-51,60-63,66-67H,17H2,1-16H3,(H,68,69)(H,70,71) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055419

(CHEMBL358582 | Isophthalic acid bis-[4-(4-carboxy-...)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)c3cccc(c3)C(=O)Oc3c(C)c(C)c(C(=O)Oc4c(C)c(C)c(C(O)=O)c(OC)c4C)c(OC)c3C)c(C)c2OC)c(C)c(C)c1C(O)=O Show InChI InChI=1S/C52H54O16/c1-21-25(5)41(29(9)43(61-13)35(21)47(53)54)67-51(59)37-23(3)27(7)39(31(11)45(37)63-15)65-49(57)33-18-17-19-34(20-33)50(58)66-40-28(8)24(4)38(46(64-16)32(40)12)52(60)68-42-26(6)22(2)36(48(55)56)44(62-14)30(42)10/h17-20H,1-16H3,(H,53,54)(H,55,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055417

(CHEMBL407586 | Thielocin A1)Show SMILES COc1c(C)c(OC(=O)c2c(C)c(C)c(OC(=O)C3=C(C)[C@@](C)(O)C4(O)Oc5c(C)c(OC)c(C(=O)Oc6c(C)c(C)c(C(O)=O)c(OC)c6C)c(C)c5C[C@@]4(C)C3=O)c(C)c2OC)c(C)c(C)c1C(O)=O |c:18| Show InChI InChI=1S/C54H60O18/c1-20-23(4)38(27(8)42(65-15)33(20)47(56)57)69-49(60)35-22(3)25(6)40(29(10)44(35)67-17)71-51(62)37-31(12)53(14,63)54(64)52(13,46(37)55)19-32-26(7)36(45(68-18)30(11)41(32)72-54)50(61)70-39-24(5)21(2)34(48(58)59)43(66-16)28(39)9/h63-64H,19H2,1-18H3,(H,56,57)(H,58,59)/t52-,53+,54?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant secretory Phospholipase A2 (group I). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50015214

(6-chloro-2-methoxy-9-acridinyl(4-diethylamino-1-me...)Show SMILES CCN(CC)CCCC(C)Nc1c2ccc(Cl)cc2nc2ccc(OC)cc12 Show InChI InChI=1S/C23H30ClN3O/c1-5-27(6-2)13-7-8-16(3)25-23-19-11-9-17(24)14-22(19)26-21-12-10-18(28-4)15-20(21)23/h9-12,14-16H,5-8,13H2,1-4H3,(H,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat secretory Phospholipase A2 (group II). |
J Med Chem 39: 5183-91 (1997)
Article DOI: 10.1021/jm960437a
BindingDB Entry DOI: 10.7270/Q2K64JQH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data