

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (RAT) | BDBM50068673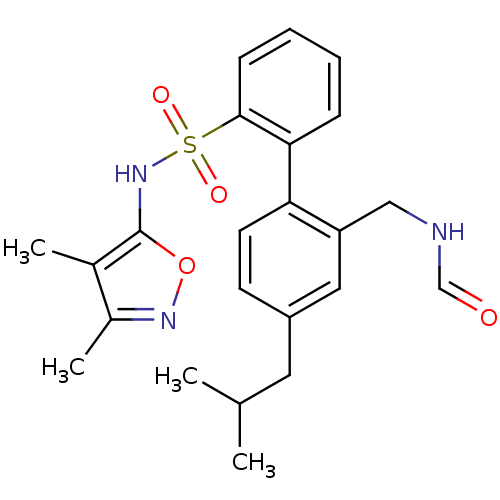 (2'-Formylaminomethyl-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068706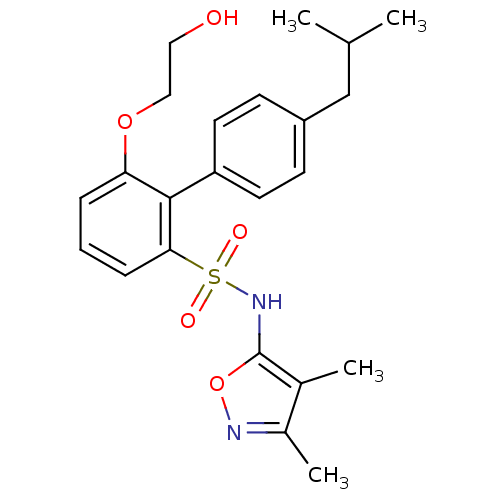 (6-(2-Hydroxy-ethoxy)-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068674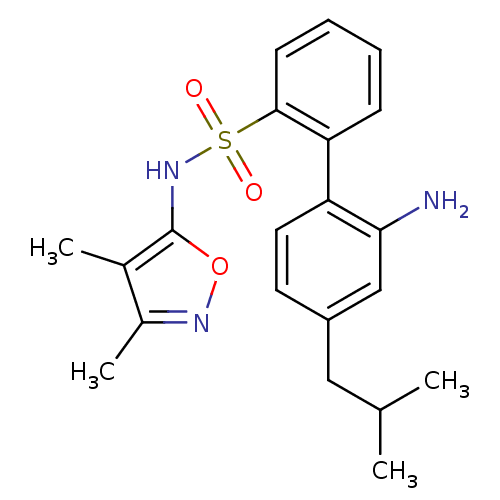 (2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068720 (6-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068713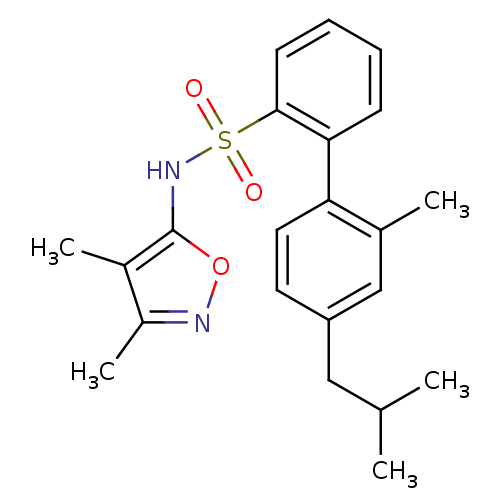 (4'-Isobutyl-2'-methyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068740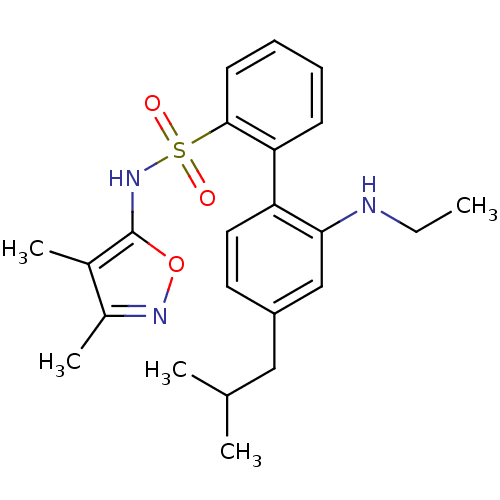 (2'-Ethylamino-4'-isobutyl-biphenyl-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068697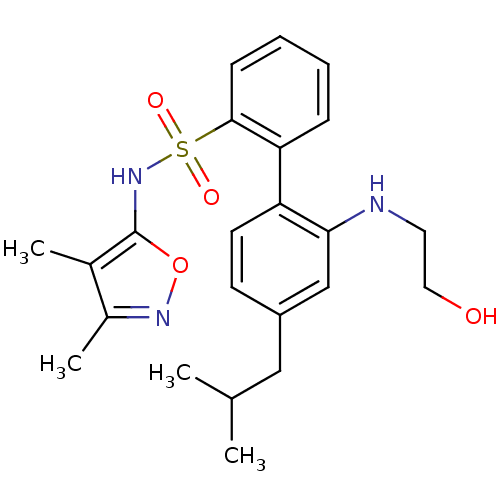 (2'-(2-Hydroxy-ethylamino)-4'-isobutyl-biphenyl-2-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068736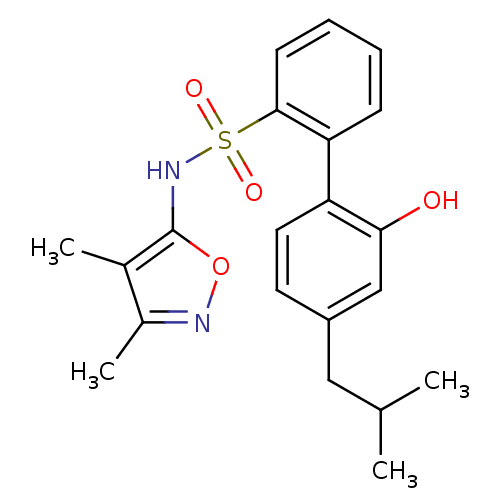 (2'-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068685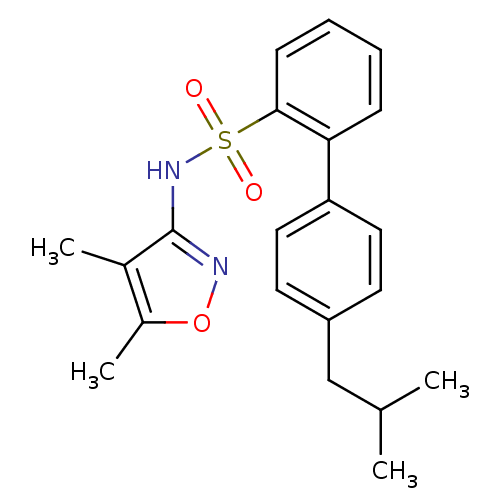 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068704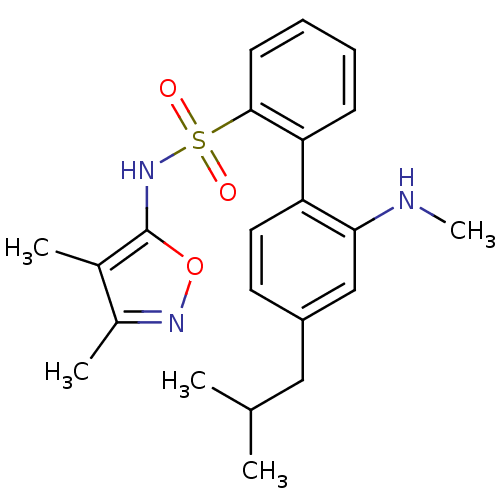 (4'-Isobutyl-2'-methylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068722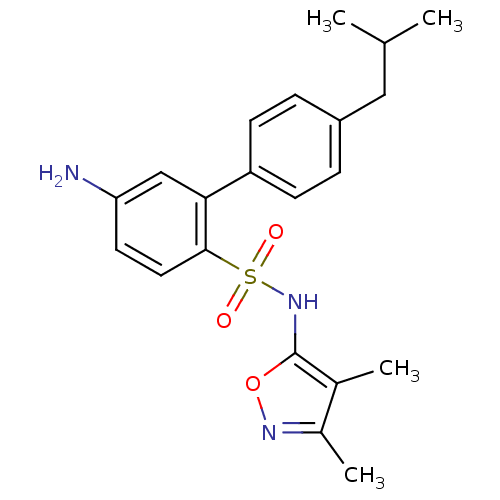 (5-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068716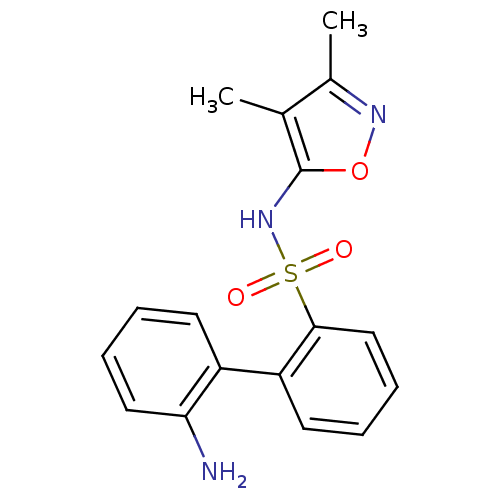 (2'-Amino-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068719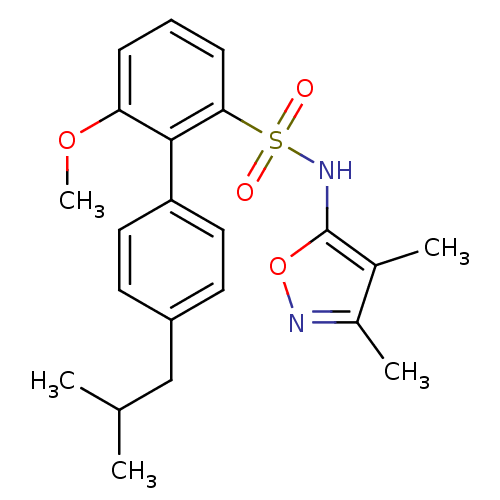 (4'-Isobutyl-6-methoxy-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068676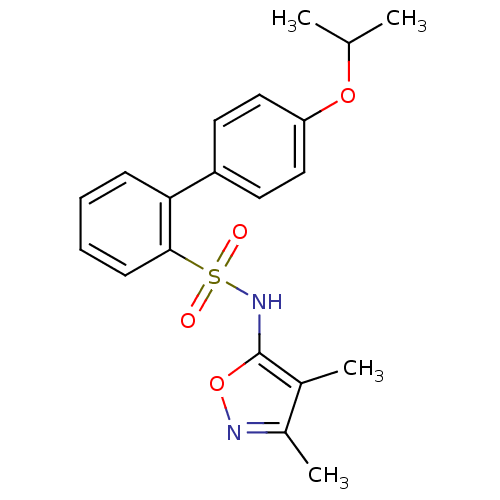 (4'-Isopropoxy-biphenyl-2-sulfonic acid (3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068717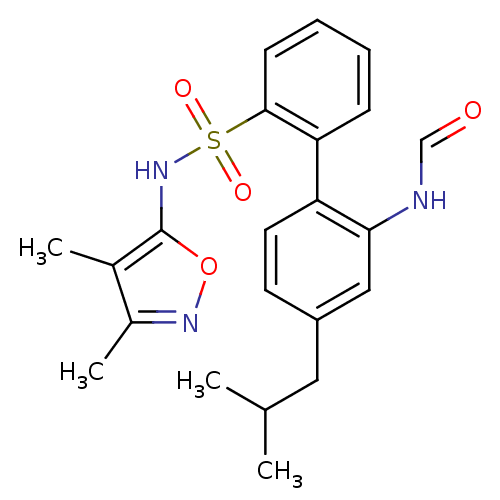 (2'-Formylamino-4'-isobutyl-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068742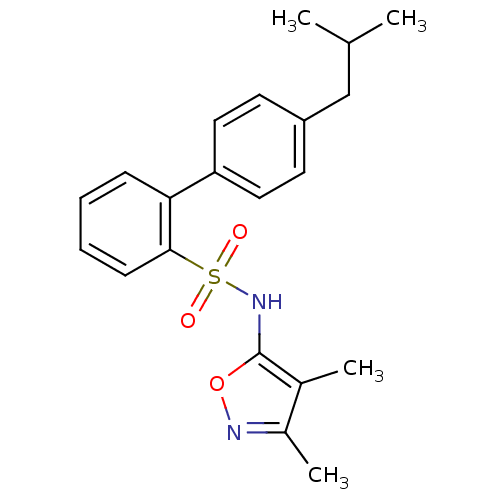 (4'-Isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068698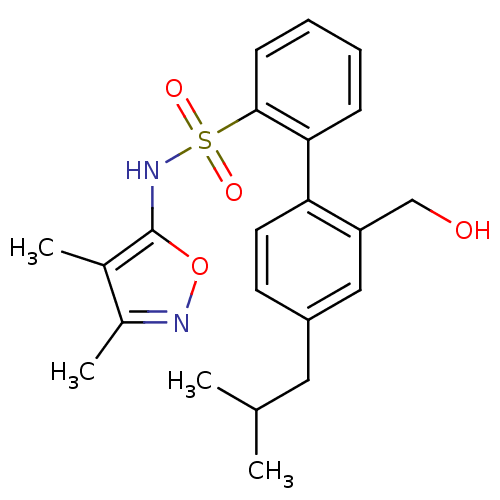 (2'-Hydroxymethyl-4'-isobutyl-biphenyl-2-sulfonic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068729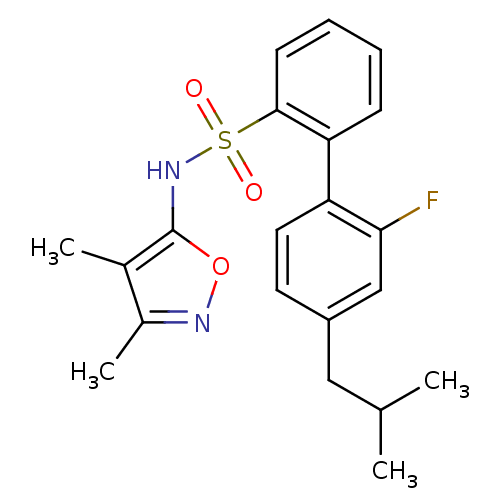 (2'-Fluoro-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068694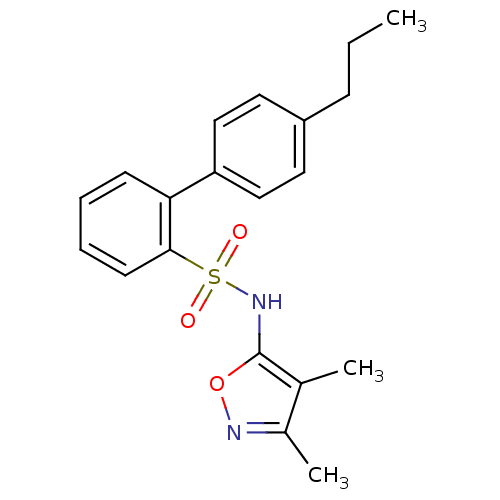 (4'-Propyl-biphenyl-2-sulfonic acid (3,4-dimethyl-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068677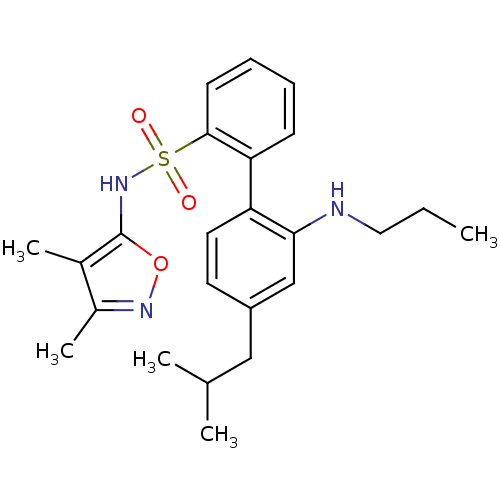 (4'-Isobutyl-2'-propylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068686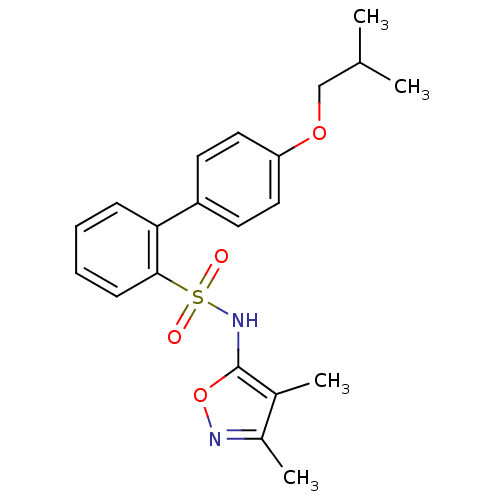 (4'-Isobutoxy-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068746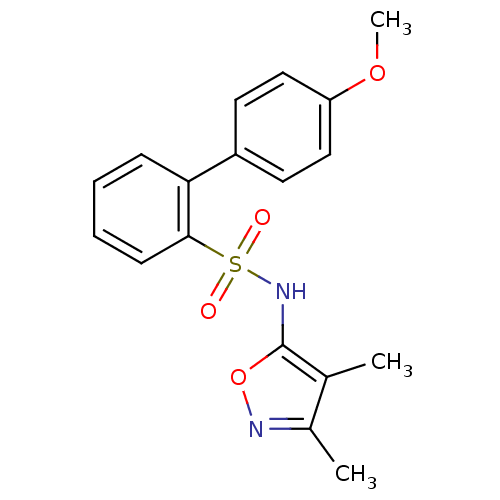 (4'-Methoxy-biphenyl-2-sulfonic acid (3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068738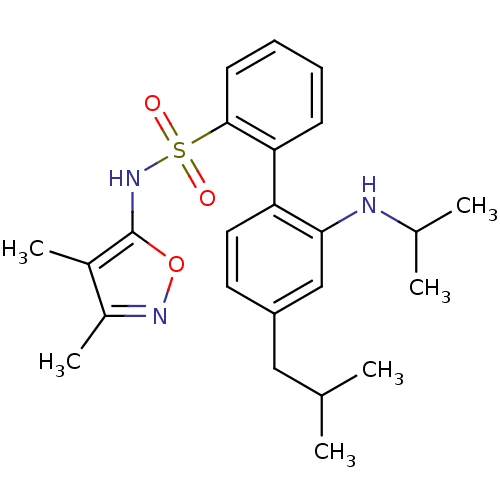 (4'-Isobutyl-2'-isopropylamino-biphenyl-2-sulfonic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068735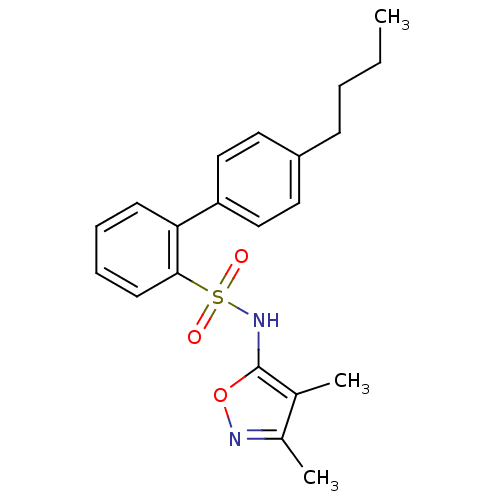 (4'-Butyl-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068731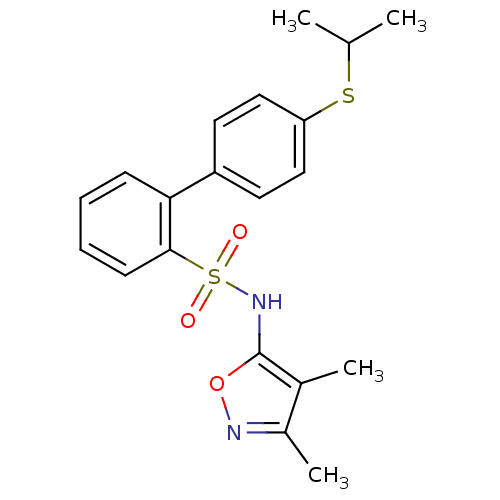 (4'-Isopropylsulfanyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068703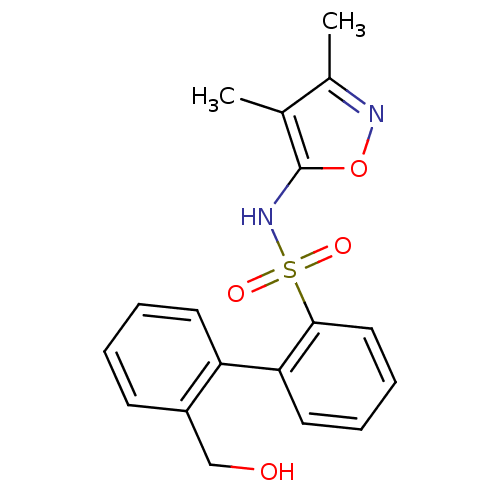 (2'-Hydroxymethyl-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50329850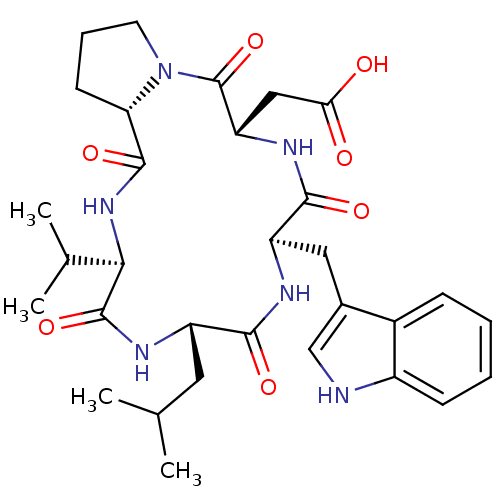 ((R)-3-Amino-4-[(S)-2-((R)-1-{(S)-1-[(R)-1-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068691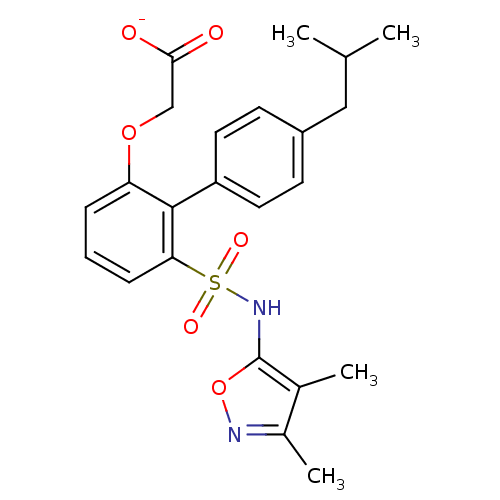 (CHEMBL149152 | Lithium; [6-(3,4-dimethyl-isoxazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068710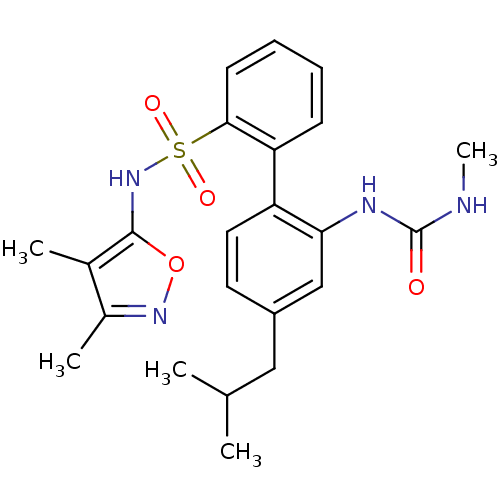 (4'-Isobutyl-2'-(3-methyl-ureido)-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068727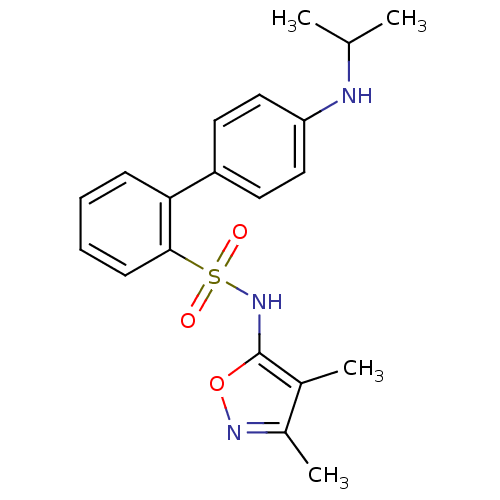 (4'-Isopropylamino-biphenyl-2-sulfonic acid (3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068705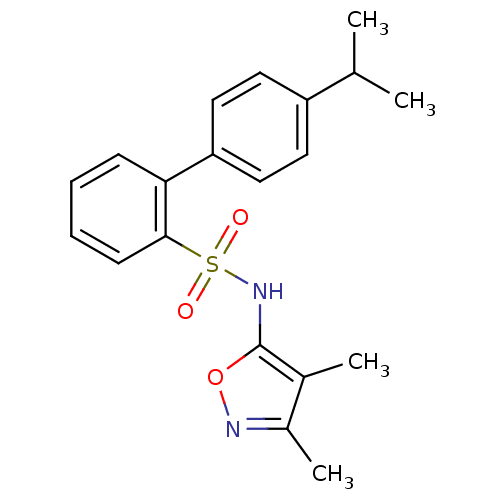 (4'-Isopropyl-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068682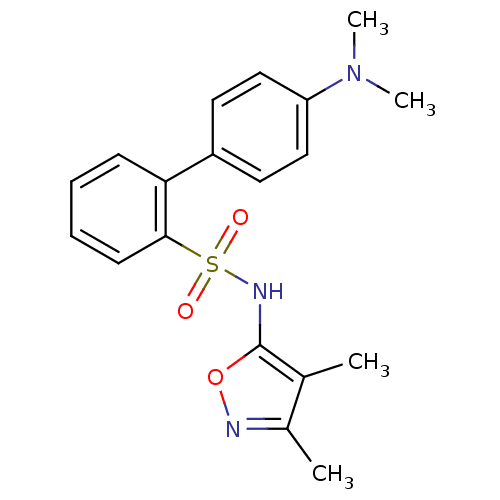 (4'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50034435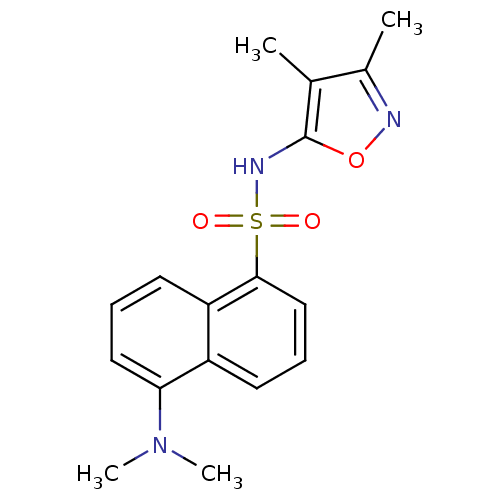 (5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068701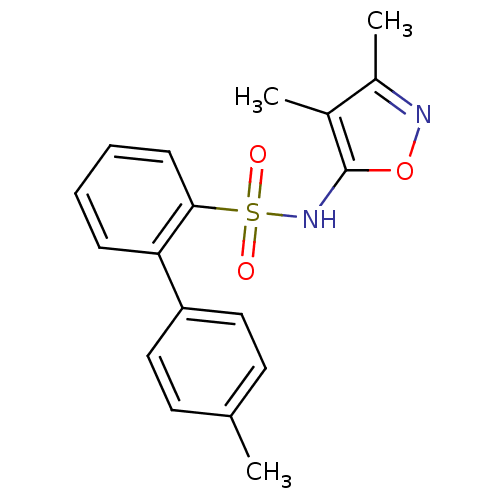 (4'-Methyl-biphenyl-2-sulfonic acid (3,4-dimethyl-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068683 (4'-(Isopropyl-methyl-amino)-biphenyl-2-sulfonic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068687 (4'-tert-Butyl-biphenyl-2-sulfonic acid (3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068680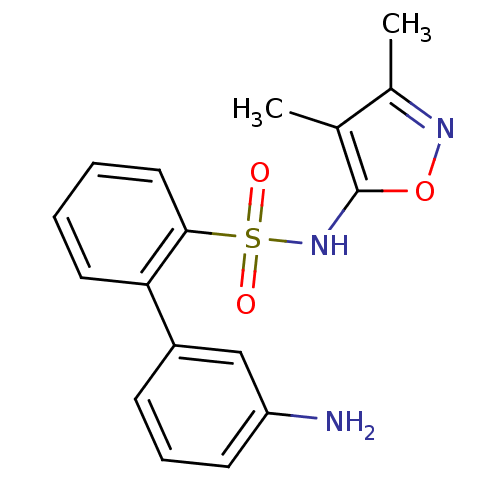 (3'-Amino-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068743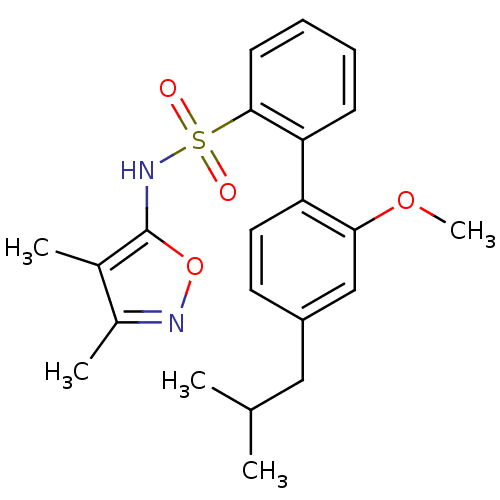 (4'-Isobutyl-2'-methoxy-biphenyl-2-sulfonic acid (3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068726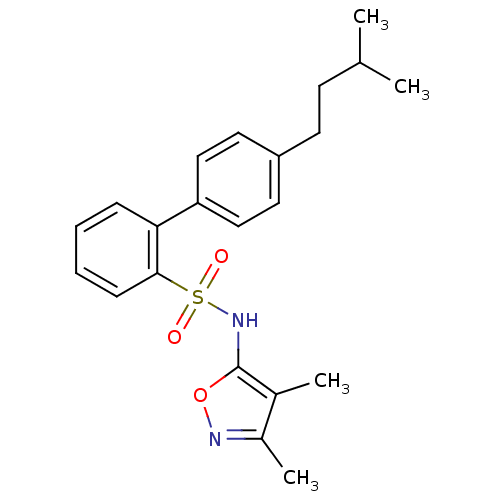 (4'-(3-Methyl-butyl)-biphenyl-2-sulfonic acid (3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068688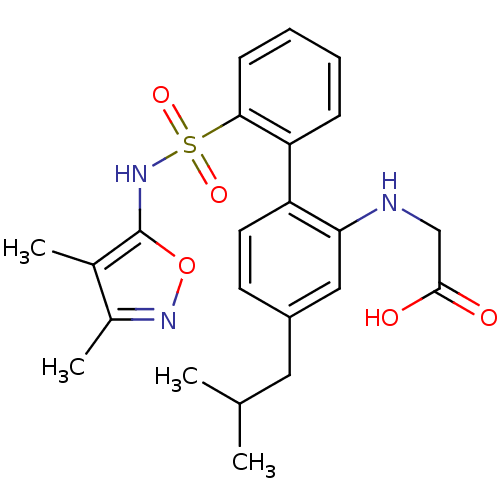 (CHEMBL357823 | [2'-(3,4-Dimethyl-isoxazol-5-ylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068700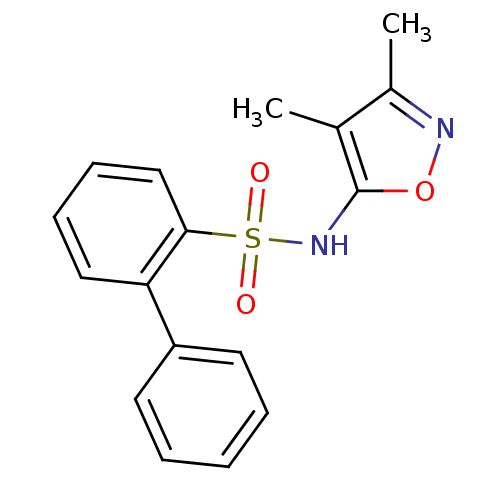 (Biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068692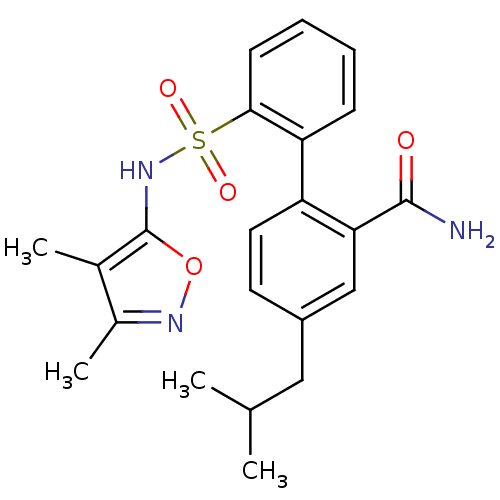 (2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-4-isobuty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068690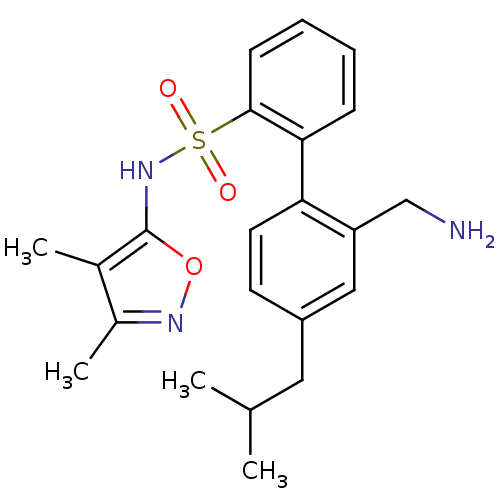 (2'-Aminomethyl-4'-isobutyl-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068741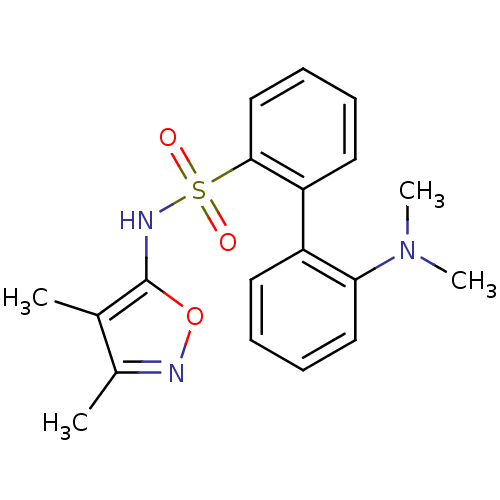 (2'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068733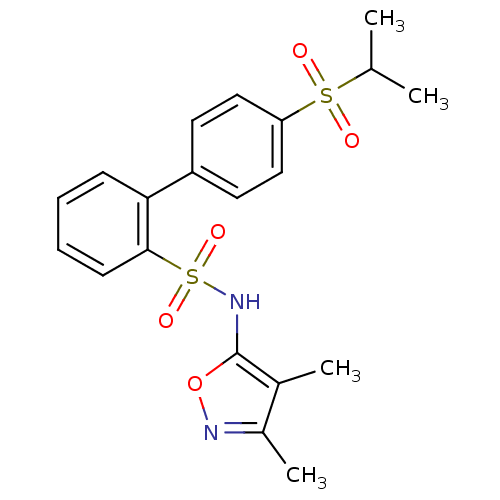 (4'-(Propane-2-sulfonyl)-biphenyl-2-sulfonic acid (...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068695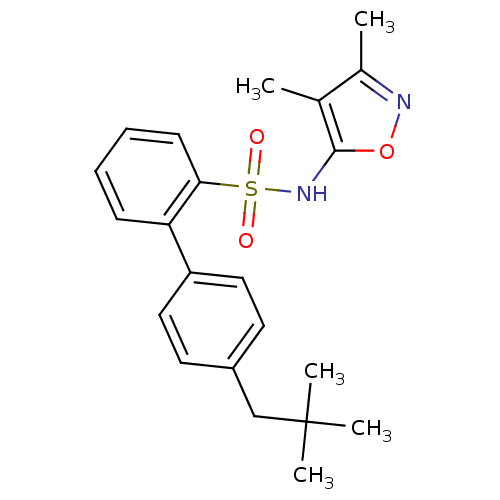 (4'-(2,2-Dimethyl-propyl)-biphenyl-2-sulfonic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068748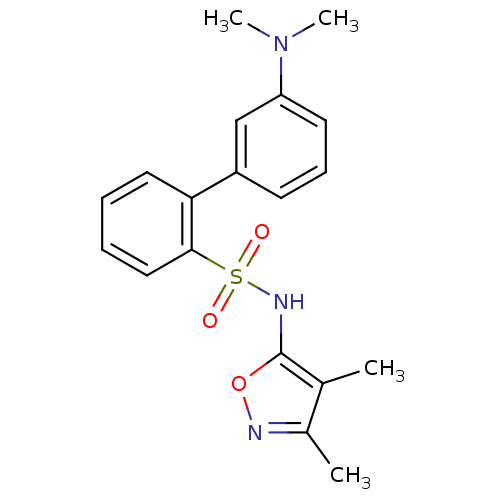 (3'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068707 (2'-Nitro-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068678 (3'-Isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068681 (4'-Benzyloxy-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068708 (CHEMBL148637 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068709 (2'-Dimethylamino-4'-isobutyl-biphenyl-2-sulfonic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068696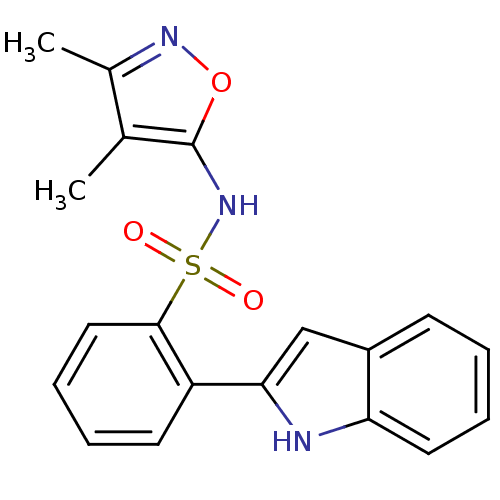 (CHEMBL148967 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068711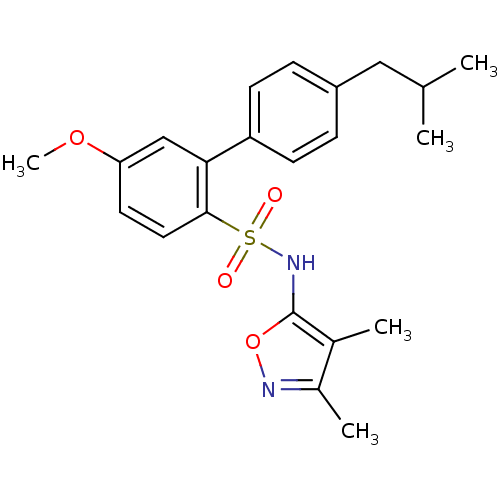 (4'-Isobutyl-5-methoxy-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068732 (4'-Isobutyl-biphenyl-2-sulfonic acid (6-chloro-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068730 (4-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068679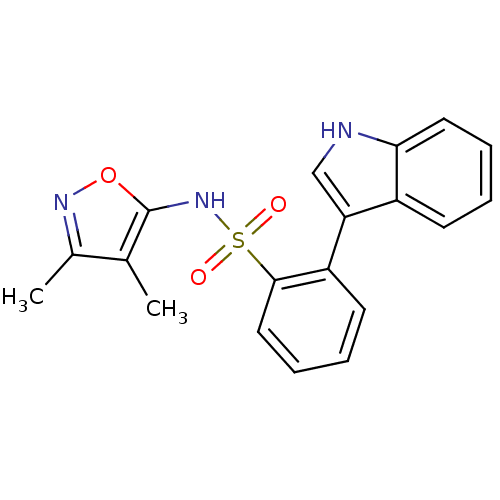 (CHEMBL357331 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068670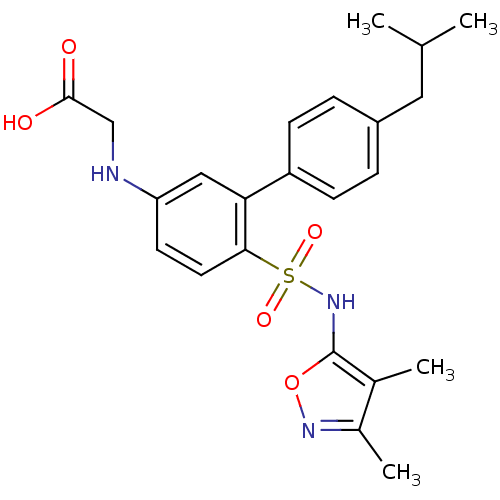 (CHEMBL358461 | [6-(3,4-Dimethyl-isoxazol-5-ylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068702 (Biphenyl-2,4'-disulfonic acid 4'-dimethylamide 2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068747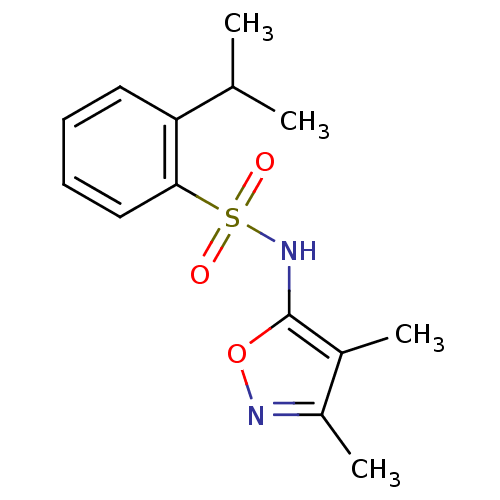 (CHEMBL342498 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068728 (CHEMBL150697 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068689 (CHEMBL149018 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068718 (CHEMBL151819 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068720 (6-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068712 (CHEMBL357789 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068671 (4'-Isobutyl-biphenyl-2-sulfonic acid (4-methyl-iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068673 (2'-Formylaminomethyl-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068684 (2-Amino-N-[6-(3,4-dimethyl-isoxazol-5-ylsulfamoyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068714 (4'-Isobutyl-3-methyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068698 (2'-Hydroxymethyl-4'-isobutyl-biphenyl-2-sulfonic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068740 (2'-Ethylamino-4'-isobutyl-biphenyl-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068672 (2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-4-isobuty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068676 (4'-Isopropoxy-biphenyl-2-sulfonic acid (3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068697 (2'-(2-Hydroxy-ethylamino)-4'-isobutyl-biphenyl-2-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068742 (4'-Isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068674 (2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068670 (CHEMBL358461 | [6-(3,4-Dimethyl-isoxazol-5-ylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068735 (4'-Butyl-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068685 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068719 (4'-Isobutyl-6-methoxy-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068715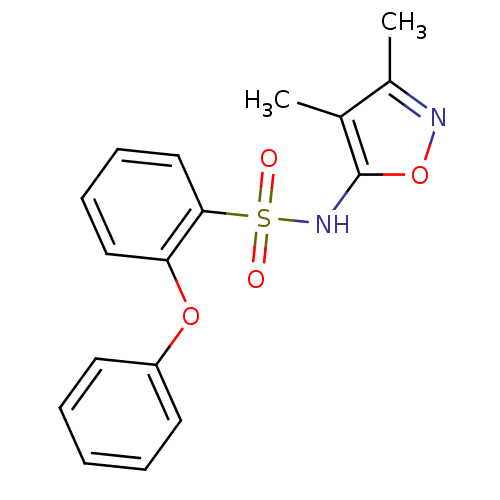 (CHEMBL147007 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068722 (5-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068704 (4'-Isobutyl-2'-methylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068706 (6-(2-Hydroxy-ethoxy)-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068739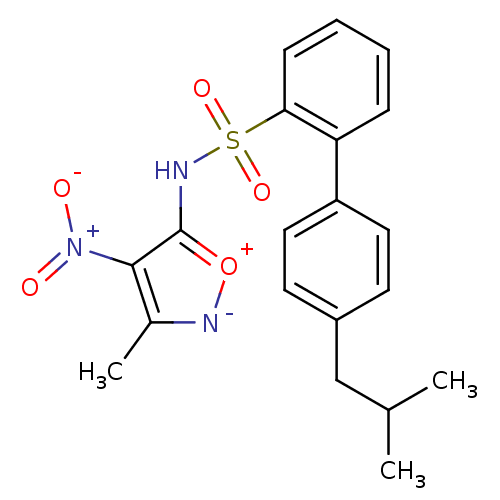 (4'-Isobutyl-biphenyl-2-sulfonic acid (3-methyl-4-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068721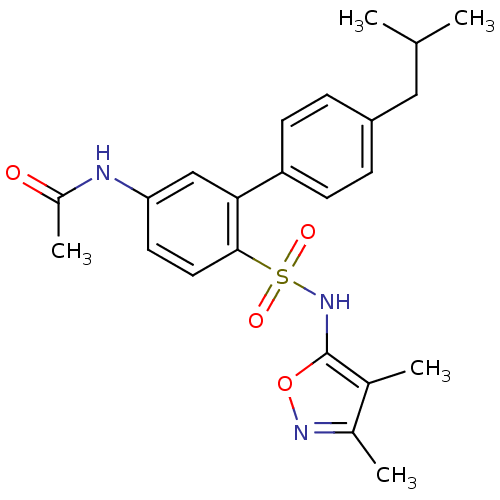 (CHEMBL150269 | N-[6-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068686 (4'-Isobutoxy-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068738 (4'-Isobutyl-2'-isopropylamino-biphenyl-2-sulfonic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068744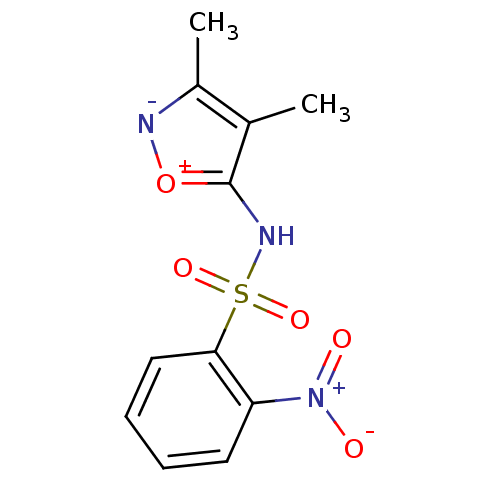 (CHEMBL151545 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068677 (4'-Isobutyl-2'-propylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068749 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,6-dimethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068691 (CHEMBL149152 | Lithium; [6-(3,4-dimethyl-isoxazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068695 (4'-(2,2-Dimethyl-propyl)-biphenyl-2-sulfonic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068699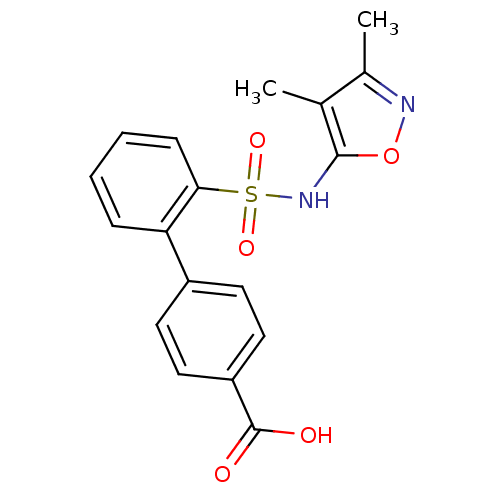 (2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068745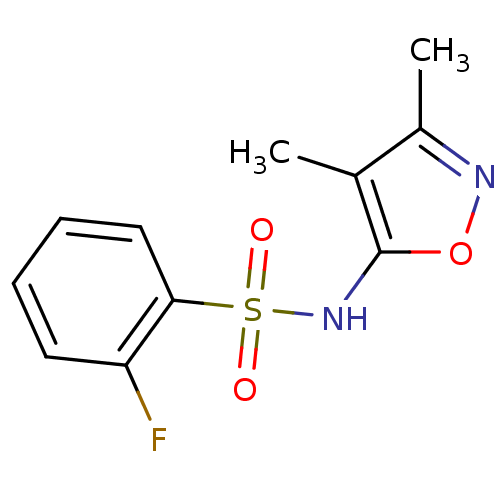 (CHEMBL78499 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068710 (4'-Isobutyl-2'-(3-methyl-ureido)-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068678 (3'-Isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068731 (4'-Isopropylsulfanyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068734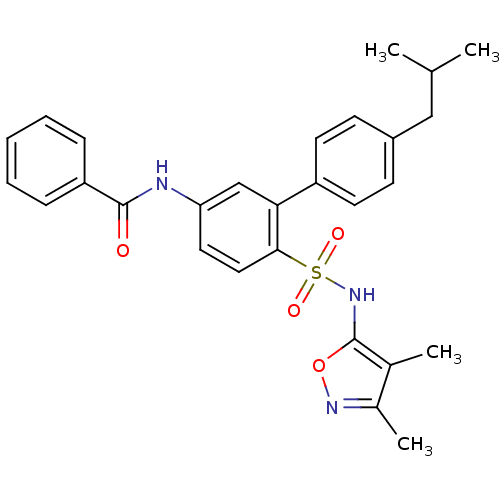 (CHEMBL343477 | N-[6-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068750 (2-Amino-N-(3,4-dimethyl-isoxazol-5-yl)-benzenesulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068682 (4'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068711 (4'-Isobutyl-5-methoxy-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068693 (4'-Isobutyl-biphenyl-2-sulfonic acid (4-benzyl-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068717 (2'-Formylamino-4'-isobutyl-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068724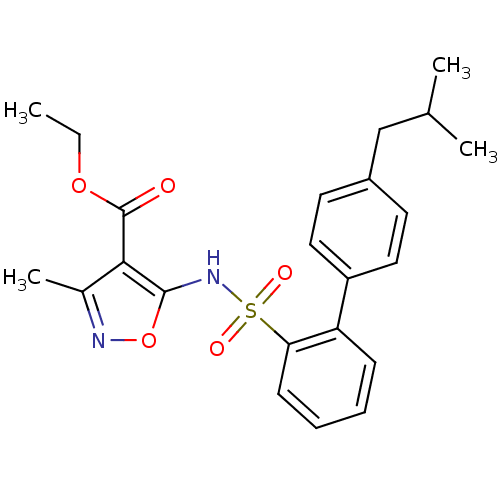 (5-(4'-Isobutyl-biphenyl-2-sulfonylamino)-3-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068684 (2-Amino-N-[6-(3,4-dimethyl-isoxazol-5-ylsulfamoyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068725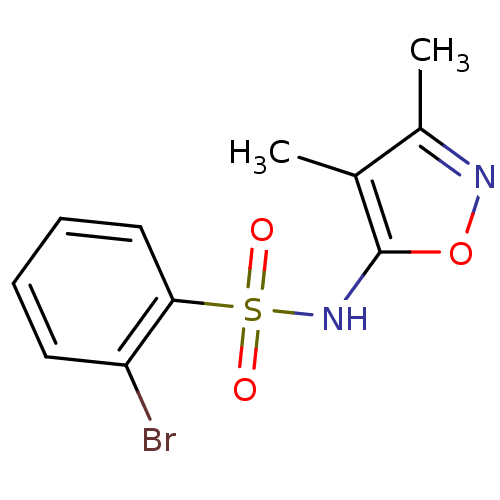 (2-Bromo-N-(3,4-dimethyl-isoxazol-5-yl)-benzenesulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle (Endothelin A receptor) was determined by rad... | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068681 (4'-Benzyloxy-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50068737 (CHEMBL357678 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit Endothelin A receptor induced contractions in rabbit carotid artery rings (ETA) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068690 (2'-Aminomethyl-4'-isobutyl-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068736 (2'-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068721 (CHEMBL150269 | N-[6-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068688 (CHEMBL357823 | [2'-(3,4-Dimethyl-isoxazol-5-ylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068692 (2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-4-isobuty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068729 (2'-Fluoro-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068694 (4'-Propyl-biphenyl-2-sulfonic acid (3,4-dimethyl-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068687 (4'-tert-Butyl-biphenyl-2-sulfonic acid (3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068727 (4'-Isopropylamino-biphenyl-2-sulfonic acid (3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068713 (4'-Isobutyl-2'-methyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068705 (4'-Isopropyl-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068714 (4'-Isobutyl-3-methyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068683 (4'-(Isopropyl-methyl-amino)-biphenyl-2-sulfonic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068723 (4-Benzyloxy-4'-isobutyl-biphenyl-2-sulfonic acid (...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068701 (4'-Methyl-biphenyl-2-sulfonic acid (3,4-dimethyl-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068730 (4-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068675 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,5,6,7-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068672 (2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-4-isobuty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068726 (4'-(3-Methyl-butyl)-biphenyl-2-sulfonic acid (3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068739 (4'-Isobutyl-biphenyl-2-sulfonic acid (3-methyl-4-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50329850 ((R)-3-Amino-4-[(S)-2-((R)-1-{(S)-1-[(R)-1-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068734 (CHEMBL343477 | N-[6-(3,4-Dimethyl-isoxazol-5-ylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068724 (5-(4'-Isobutyl-biphenyl-2-sulfonylamino)-3-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068732 (4'-Isobutyl-biphenyl-2-sulfonic acid (6-chloro-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068749 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,6-dimethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068743 (4'-Isobutyl-2'-methoxy-biphenyl-2-sulfonic acid (3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50068696 (CHEMBL148967 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit ET-1 induced contractions in rabbit carotid artery rings (Endothelin B receptor) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068671 (4'-Isobutyl-biphenyl-2-sulfonic acid (4-methyl-iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068709 (2'-Dimethylamino-4'-isobutyl-biphenyl-2-sulfonic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068733 (4'-(Propane-2-sulfonyl)-biphenyl-2-sulfonic acid (...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50034435 (5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068741 (2'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068748 (3'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068680 (3'-Amino-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068723 (4-Benzyloxy-4'-isobutyl-biphenyl-2-sulfonic acid (...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50068718 (CHEMBL151819 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit ET-1 induced contractions in rabbit carotid artery rings (Endothelin B receptor) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068716 (2'-Amino-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50068708 (CHEMBL148637 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit ET-1 induced contractions in rabbit carotid artery rings (Endothelin B receptor) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068702 (Biphenyl-2,4'-disulfonic acid 4'-dimethylamide 2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068703 (2'-Hydroxymethyl-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068699 (2'-(3,4-Dimethyl-isoxazol-5-ylsulfamoyl)-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50068737 (CHEMBL357678 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit ET-1 induced contractions in rabbit carotid artery rings (Endothelin B receptor) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50068712 (CHEMBL357789 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit ET-1 induced contractions in rabbit carotid artery rings (Endothelin B receptor) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50068679 (CHEMBL357331 | N-(3,4-Dimethyl-isoxazol-5-yl)-2-(1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit ET-1 induced contractions in rabbit carotid artery rings (Endothelin B receptor) was determined in an in vitro functional assay. | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068675 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,5,6,7-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068693 (4'-Isobutyl-biphenyl-2-sulfonic acid (4-benzyl-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068707 (2'-Nitro-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068700 (Biphenyl-2-sulfonic acid (3,4-dimethyl-isoxazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (RAT) | BDBM50068746 (4'-Methoxy-biphenyl-2-sulfonic acid (3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]-ET-1 to membranes prepared from A10 rat cerebellum (Endothelin B receptor) | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||