

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125036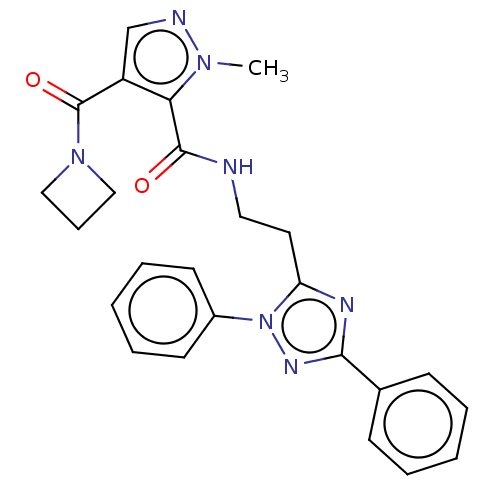 (US8772510, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125038 (US8772510, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125040 (Roche-Dataset for PDE10A, Compound 21 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125041 (Roche-Dataset for PDE10A, Compound 0 | US8772510, ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125039 (US8772510, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125012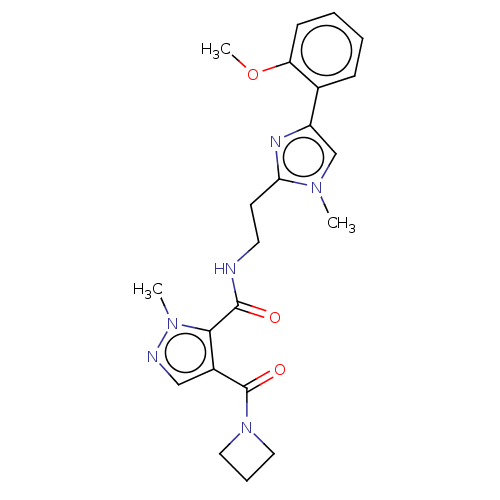 (Roche-Dataset for PDE10A, Compound 130 | US8772510...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125046 (Roche-Dataset for PDE10A, Compound 22 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125013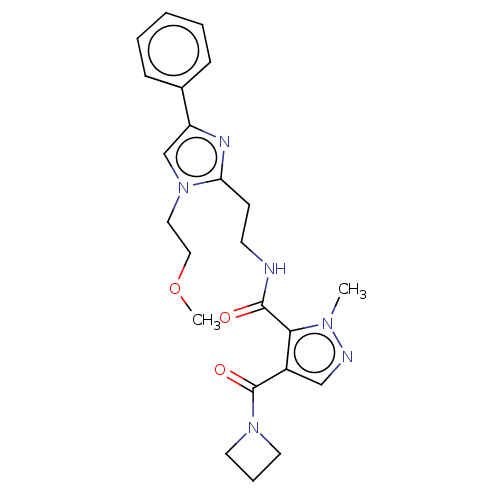 (Roche-Dataset for PDE10A, Compound 10 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125037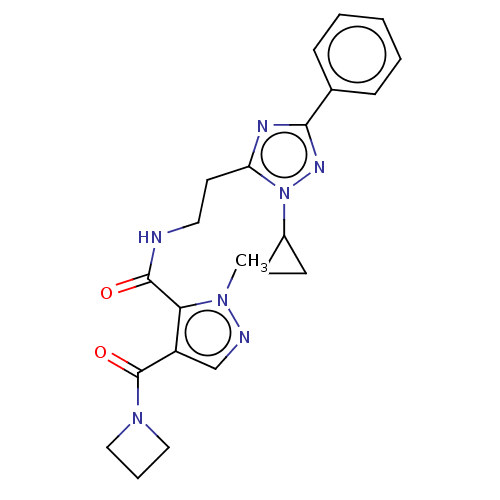 (Roche-Dataset for PDE10A, Compound 78 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125026 (Roche-Dataset for PDE10A, Compound 30 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125017 (Roche-Dataset for PDE10A, Compound 267 | US8772510...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124988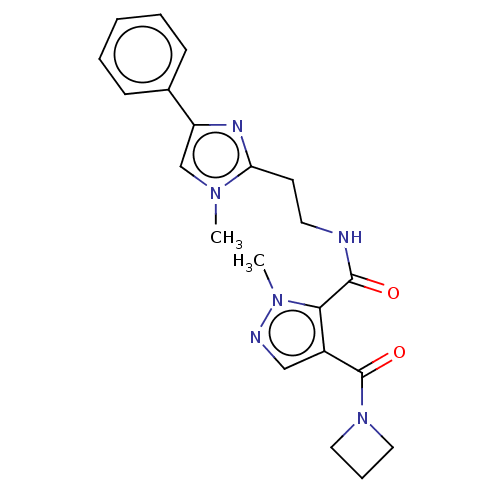 (US8772510, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125016 (Roche-Dataset for PDE10A, Compound 32 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125043 (Roche-Dataset for PDE10A, Compound 80 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125008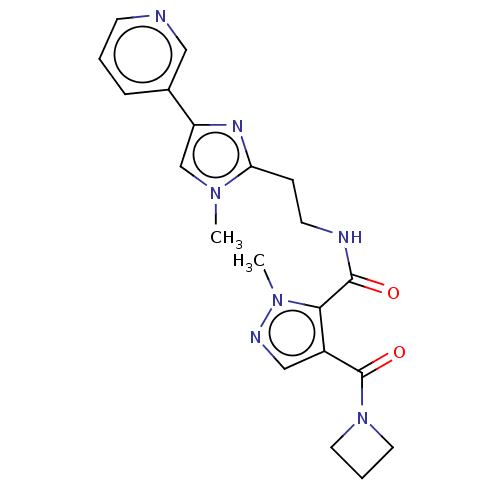 (Roche-Dataset for PDE10A, Compound 151 | US8772510...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125005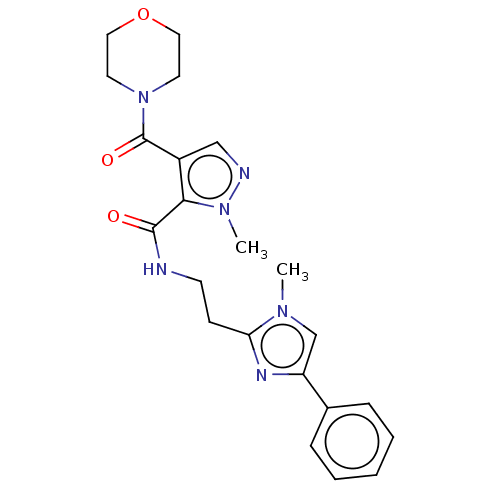 (US8772510, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124985 (US8772510, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125021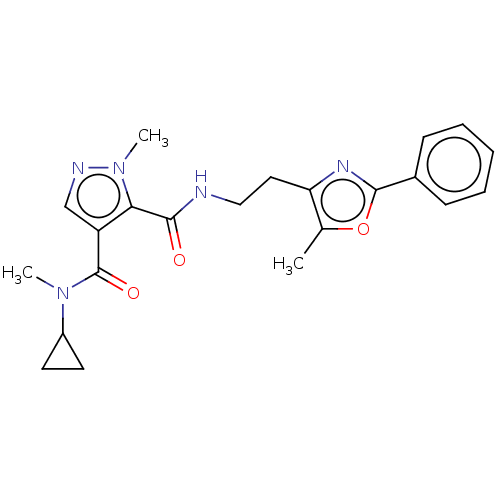 (US8772510, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125001 (US8772510, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125028 (US8772510, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124983 (US8772510, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125024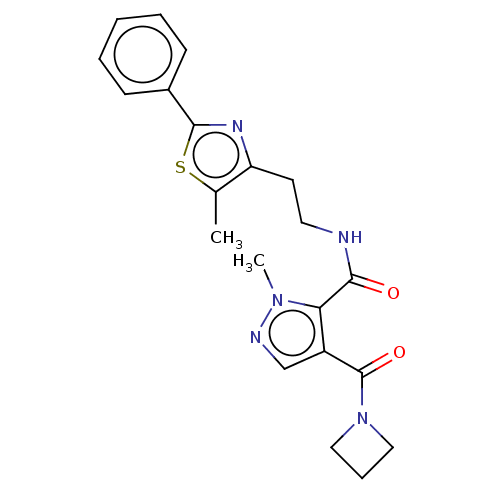 (US8772510, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125020 (US8772510, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125009 (US8772510, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124997 (US8772510, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125003 (US8772510, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125007 (US8772510, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125019 (US8772510, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125034 (US8772510, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125010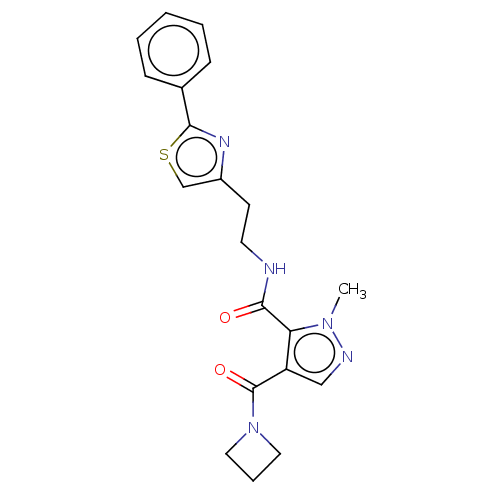 (US8772510, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124992 (US8772510, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125011 (Roche-Dataset for PDE10A, Compound 67 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125030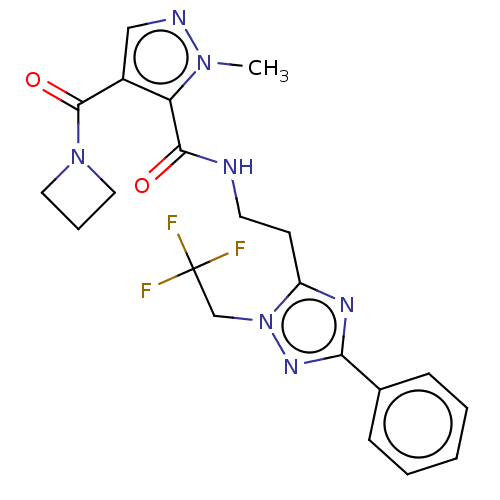 (Roche-Dataset for PDE10A, Compound 49 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125018 (US8772510, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125042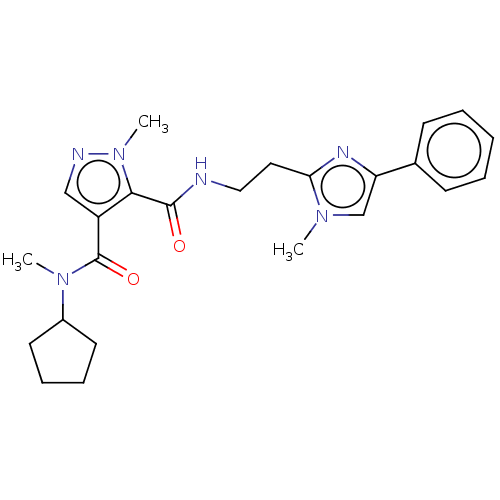 (Roche-Dataset for PDE10A, Compound 68 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 54.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125044 (Roche-Dataset for PDE10A, Compound 363 | US8772510...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 56.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124984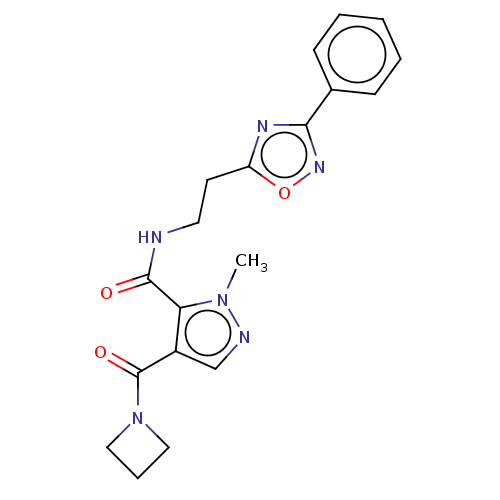 (US8772510, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124993 (US8772510, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125033 (Roche-Dataset for PDE10A, Compound 169 | US8772510...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 73.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125004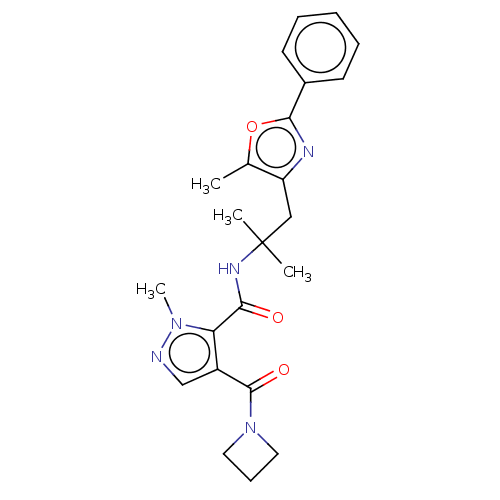 (US8772510, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125014 (Roche-Dataset for PDE10A, Compound 61 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124982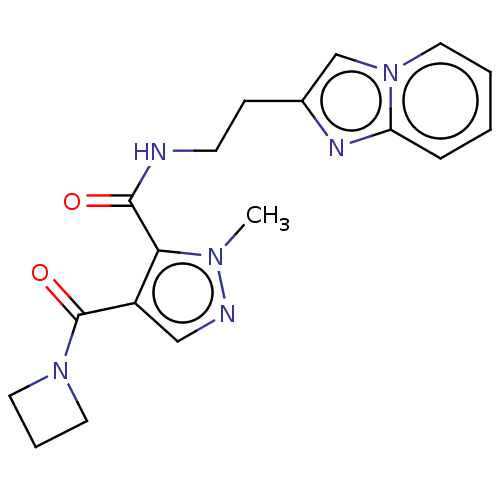 (US8772510, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 86.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124999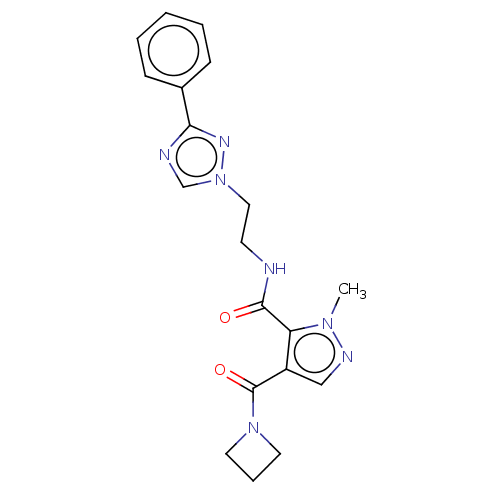 (US8772510, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125025 (US8772510, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125032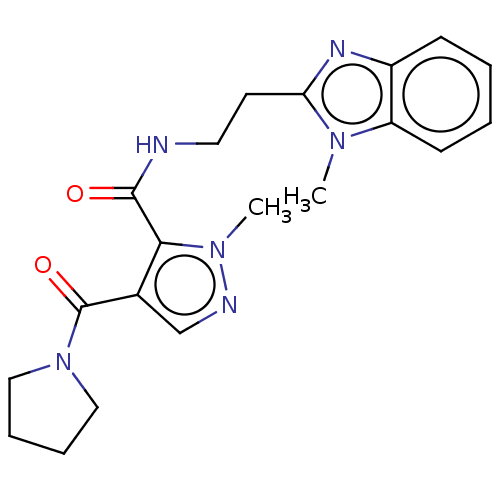 (US8772510, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125035 (US8772510, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124990 (US8772510, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125002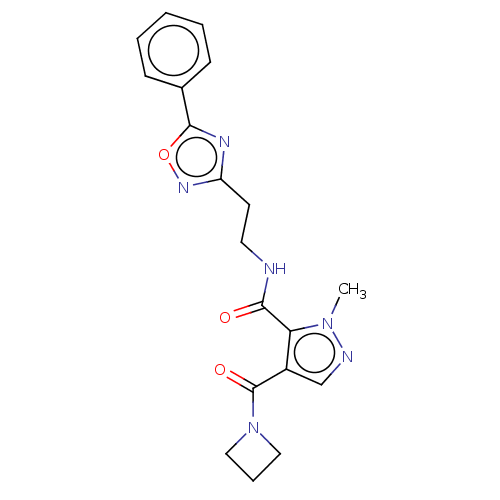 (US8772510, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124996 (US8772510, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125000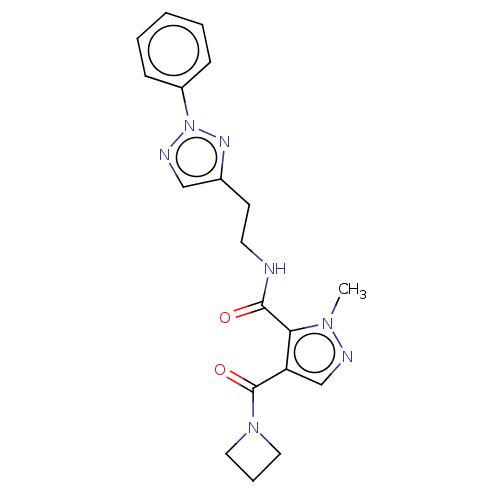 (US8772510, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125022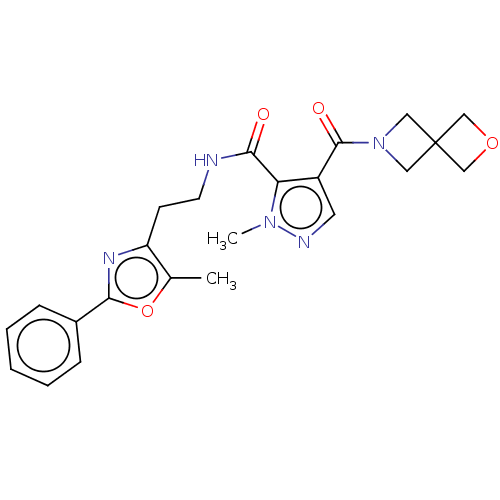 (US8772510, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125045 (US8772510, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125023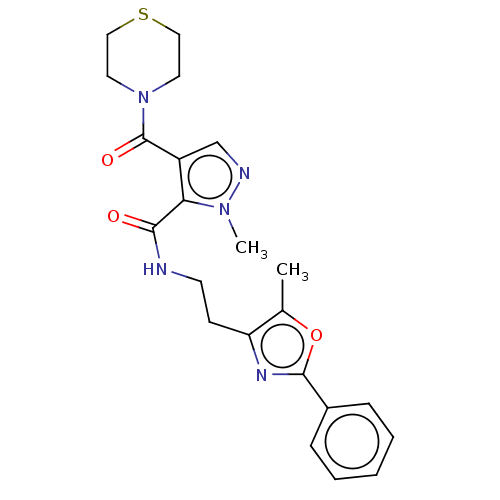 (US8772510, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125015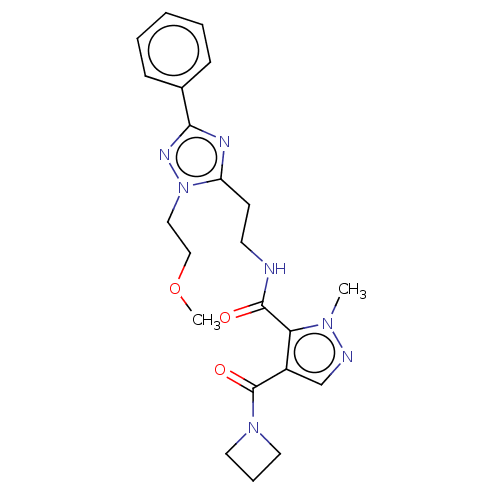 (Roche-Dataset for PDE10A, Compound 29 | US8772510,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125029 (US8772510, 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124987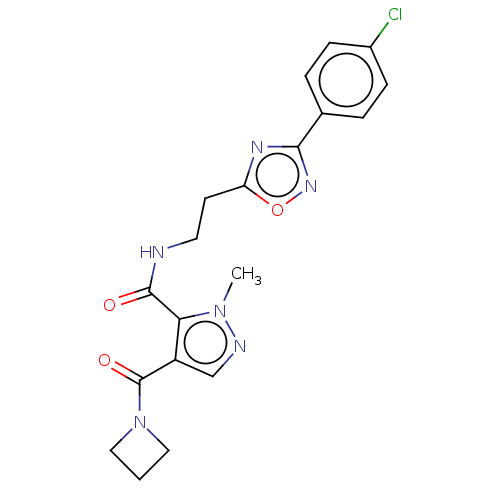 (US8772510, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125031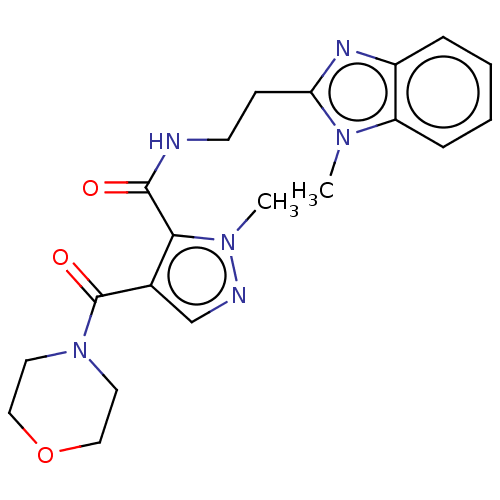 (US8772510, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124986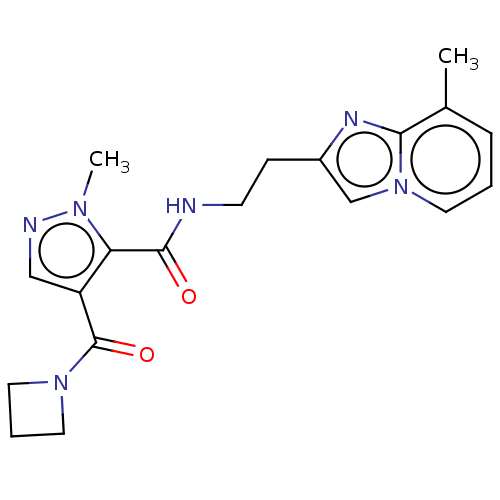 (US8772510, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124994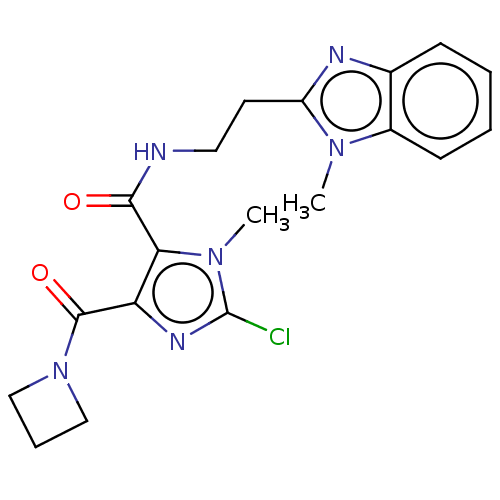 (US8772510, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 659 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125027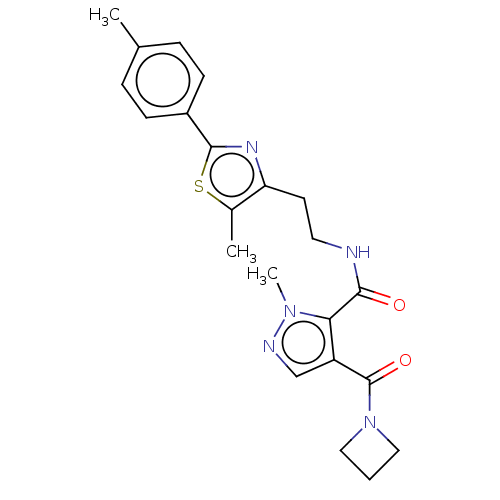 (US8772510, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 661 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124995 (US8772510, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 714 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM125006 (US8772510, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 763 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124989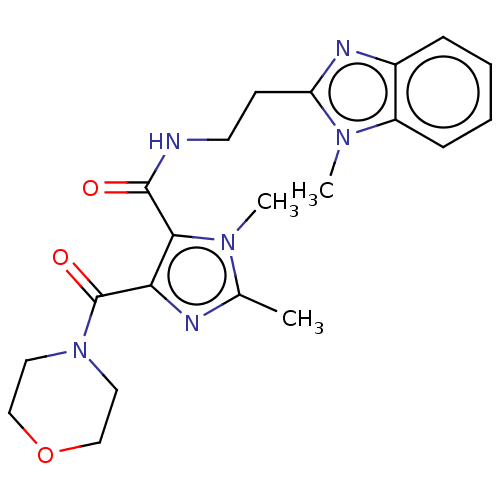 (US8772510, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124998 (US8772510, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM124991 (US8772510, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description PDE10 activity of the compounds of the present invention was determined using a Scintillation Proximity Assay (SPA)-based method similar to the one p... | US Patent US8772510 (2014) BindingDB Entry DOI: 10.7270/Q2MG7N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||