

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381082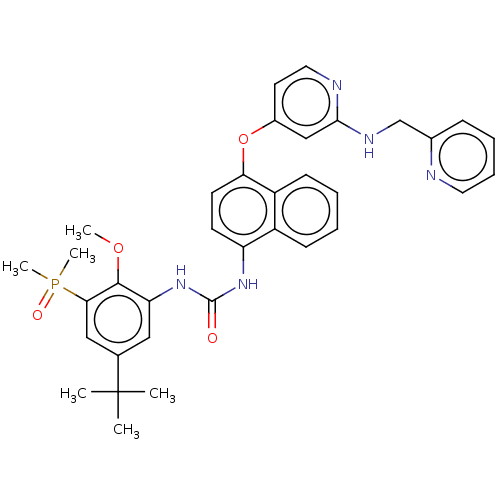 (US9890185, Example 38(e)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381166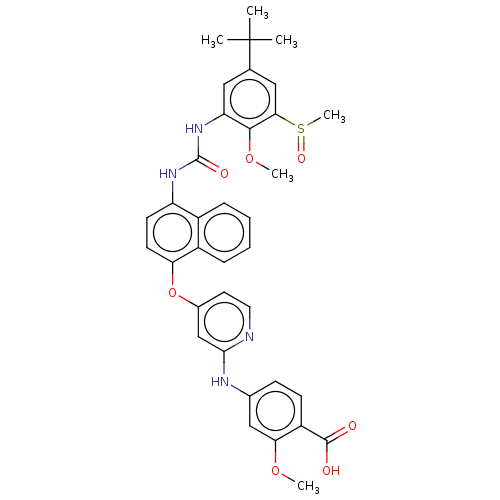 (US9890185, Example 79(g)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381071 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381094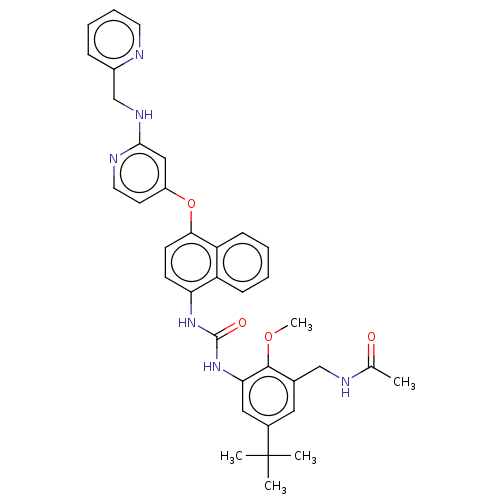 (US9890185, Example 38(q)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381123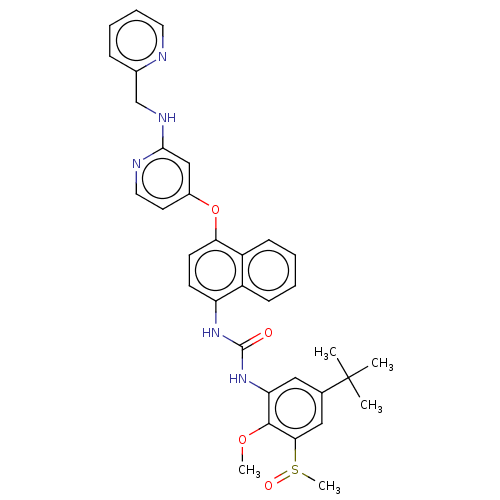 (1-(5-(tert-Butyl)-2-methoxy-3-(methylsulfinyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381092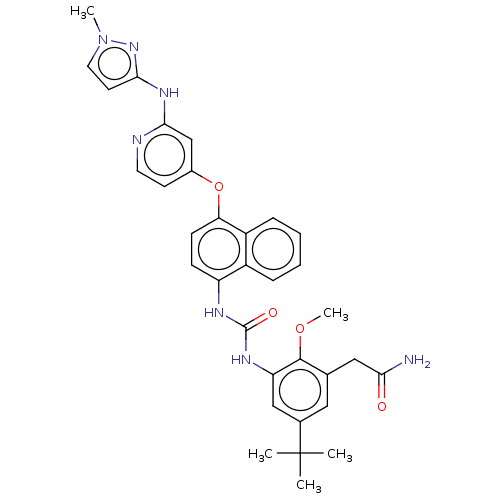 (US9890185, Example 38(o)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381084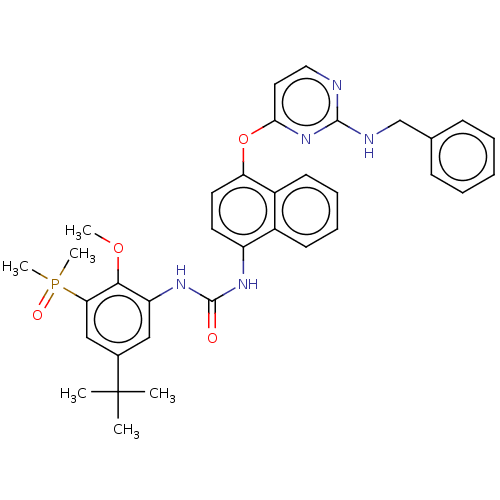 (US9890185, Example 38(g)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM380997 (US9890185, Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM380998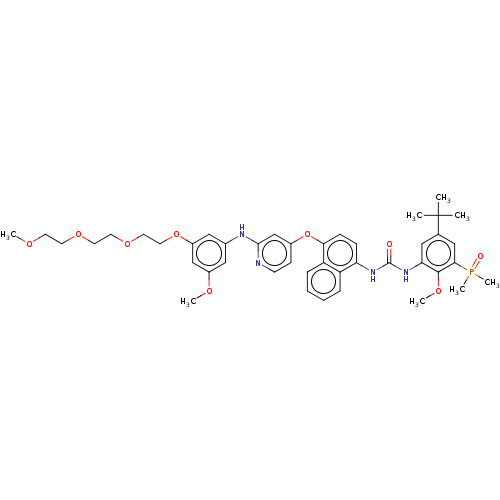 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381150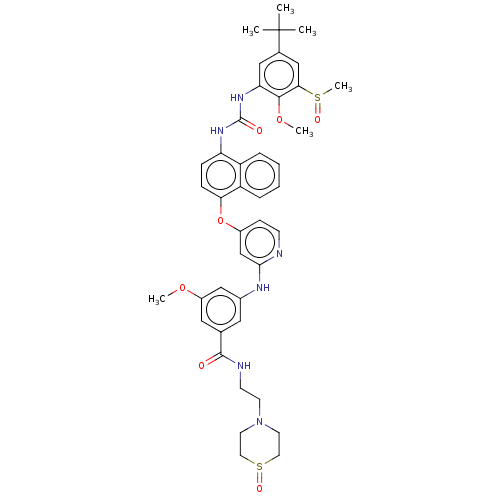 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381134 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381126 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381080 (US9890185, Example 38(c)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381149 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381081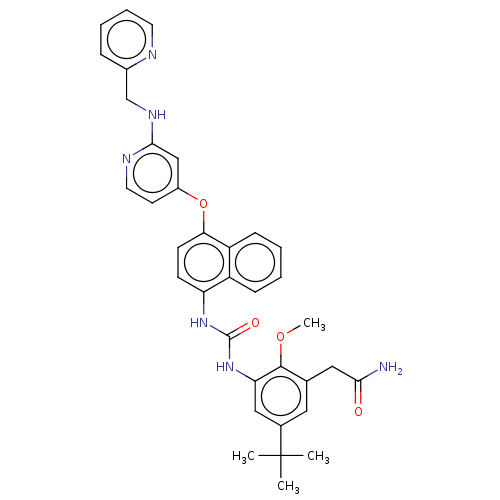 (US9890185, Example 38(d)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381098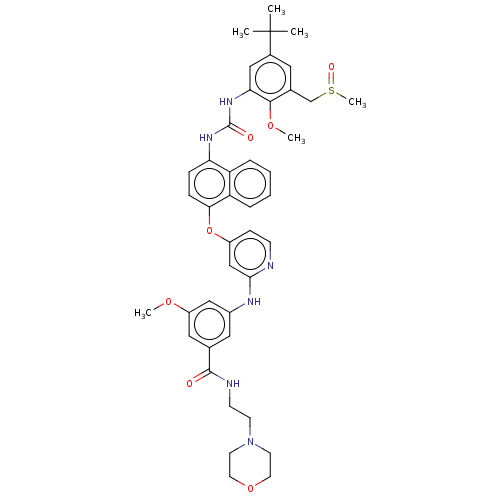 (US9890185, Example 38(u)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381012 (US9890185, Example 15(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381004 (5-(tert-Butyl)-2-methoxy-3-(3-(4-((2-((3-methoxy-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381152 (3-((4-((4-(3-(3-(Acetamidomethyl)-5-(tert-butyl)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381057 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381098 (US9890185, Example 38(u)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381146 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381102 (US9890185, Example 38(y)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381013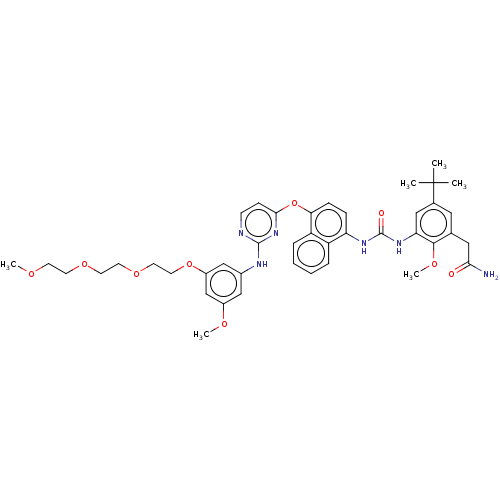 (US9890185, Example 15(c)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381002 (1-(5-(tert-Butyl)-3-((dimethylphosphoryl)methoxy)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381020 (US9890185, Example 15(j)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381006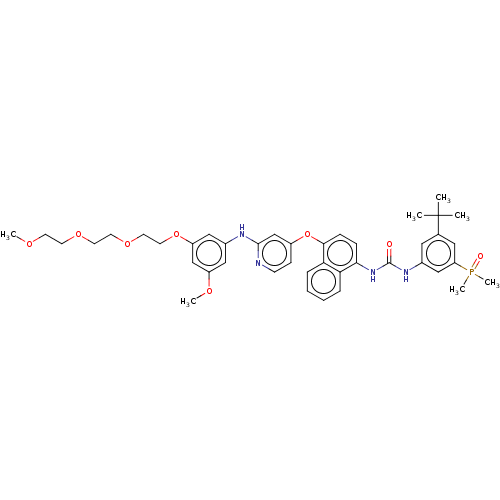 (1-(3-(tert-Butyl)-5-(dimethylphosphoryl)phenyl)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381150 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381146 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381166 (US9890185, Example 79(g)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381158 (3-((4-((4-(3-(5-(tert-Butyl)-3-((2-(dimethylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381149 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381020 (US9890185, Example 15(j)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381158 (3-((4-((4-(3-(5-(tert-Butyl)-3-((2-(dimethylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381153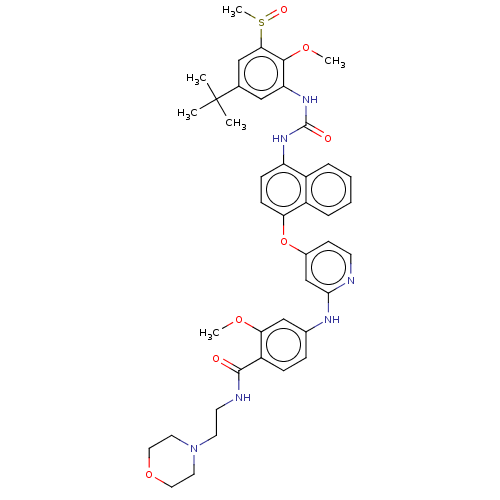 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381126 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381102 (US9890185, Example 38(y)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381134 (3-((4-((4-(3-(3-(2-Amino-2-oxoethyl)-5-(tert-butyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381117 (US9890185, Example 38(an)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381068 (1-(5-(tert-Butyl)-3-((dimethylphosphoryl)methyl)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381127 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381167 (US9890185, Example 79(m)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381129 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381152 (3-((4-((4-(3-(3-(Acetamidomethyl)-5-(tert-butyl)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381127 (3-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM381152 (3-((4-((4-(3-(3-(Acetamidomethyl)-5-(tert-butyl)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 2: The inhibitory activities of compounds of the invention against p38MAPKγ (MAPK12: Invitrogen), are evaluated in a similar fashion to t... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM381075 (1-(5-(tert-Butyl)-3-(dimethylphosphoryl)-2-methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM381080 (US9890185, Example 38(c)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon | Assay Description Method 1: The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determinin... | J Med Chem 51: 5680-9 (2008) BindingDB Entry DOI: 10.7270/Q2FB558N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 468 total ) | Next | Last >> |