| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50538099 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1972162 (CHEMBL4604980) |
|---|
| IC50 | >25000±n/a nM |
|---|
| Citation |  Liu, YA; Jin, Q; Zou, Y; Ding, Q; Yan, S; Wang, Z; Hao, X; Nguyen, B; Zhang, X; Pan, J; Mo, T; Jacobsen, K; Lam, T; Wu, TY; Petrassi, HM; Bursulaya, B; DiDonato, M; Gordon, WP; Liu, B; Baaten, J; Hill, R; Nguyen-Tran, V; Qiu, M; Zhang, YQ; Kamireddy, A; Espinola, S; Deaton, L; Ha, S; Harb, G; Jia, Y; Li, J; Shen, W; Schumacher, AM; Colman, K; Glynne, R; Pan, S; McNamara, P; Laffitte, B; Meeusen, S; Molteni, V; Loren, J Selective DYRK1A Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative GNF2133. J Med Chem63:2958-2973 (2020) [PubMed] Article Liu, YA; Jin, Q; Zou, Y; Ding, Q; Yan, S; Wang, Z; Hao, X; Nguyen, B; Zhang, X; Pan, J; Mo, T; Jacobsen, K; Lam, T; Wu, TY; Petrassi, HM; Bursulaya, B; DiDonato, M; Gordon, WP; Liu, B; Baaten, J; Hill, R; Nguyen-Tran, V; Qiu, M; Zhang, YQ; Kamireddy, A; Espinola, S; Deaton, L; Ha, S; Harb, G; Jia, Y; Li, J; Shen, W; Schumacher, AM; Colman, K; Glynne, R; Pan, S; McNamara, P; Laffitte, B; Meeusen, S; Molteni, V; Loren, J Selective DYRK1A Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative GNF2133. J Med Chem63:2958-2973 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50538099 |
|---|
| n/a |
|---|
| Name | BDBM50538099 |
|---|
| Synonyms: | CHEMBL4636064 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H30N6O2 |
|---|
| Mol. Mass. | 434.534 |
|---|
| SMILES | CCN1CCN(CC1)C(=O)Nc1cc(ccn1)-c1cn(C2CCOCC2)c2cnccc12 |
|---|
| Structure | 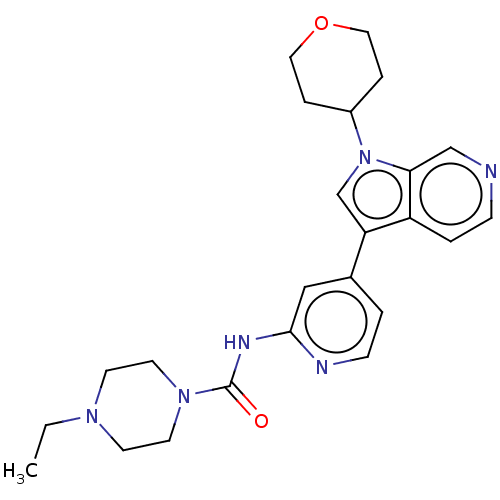
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liu, YA; Jin, Q; Zou, Y; Ding, Q; Yan, S; Wang, Z; Hao, X; Nguyen, B; Zhang, X; Pan, J; Mo, T; Jacobsen, K; Lam, T; Wu, TY; Petrassi, HM; Bursulaya, B; DiDonato, M; Gordon, WP; Liu, B; Baaten, J; Hill, R; Nguyen-Tran, V; Qiu, M; Zhang, YQ; Kamireddy, A; Espinola, S; Deaton, L; Ha, S; Harb, G; Jia, Y; Li, J; Shen, W; Schumacher, AM; Colman, K; Glynne, R; Pan, S; McNamara, P; Laffitte, B; Meeusen, S; Molteni, V; Loren, J Selective DYRK1A Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative GNF2133. J Med Chem63:2958-2973 (2020) [PubMed] Article
Liu, YA; Jin, Q; Zou, Y; Ding, Q; Yan, S; Wang, Z; Hao, X; Nguyen, B; Zhang, X; Pan, J; Mo, T; Jacobsen, K; Lam, T; Wu, TY; Petrassi, HM; Bursulaya, B; DiDonato, M; Gordon, WP; Liu, B; Baaten, J; Hill, R; Nguyen-Tran, V; Qiu, M; Zhang, YQ; Kamireddy, A; Espinola, S; Deaton, L; Ha, S; Harb, G; Jia, Y; Li, J; Shen, W; Schumacher, AM; Colman, K; Glynne, R; Pan, S; McNamara, P; Laffitte, B; Meeusen, S; Molteni, V; Loren, J Selective DYRK1A Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative GNF2133. J Med Chem63:2958-2973 (2020) [PubMed] Article