

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50243232 (CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50243232 (CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50247192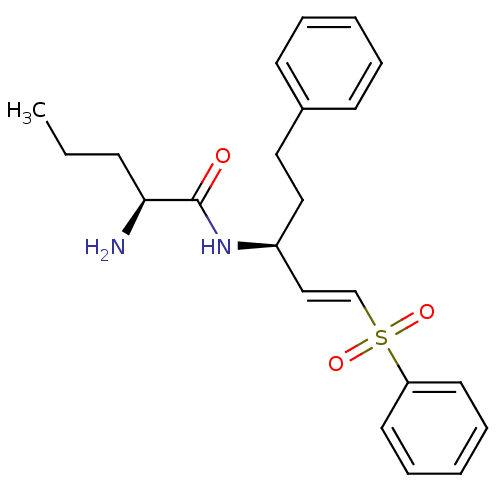 ((S)-2-amino-N-((S)-5-phenyl-1-(phenylsulfonyl)pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50186088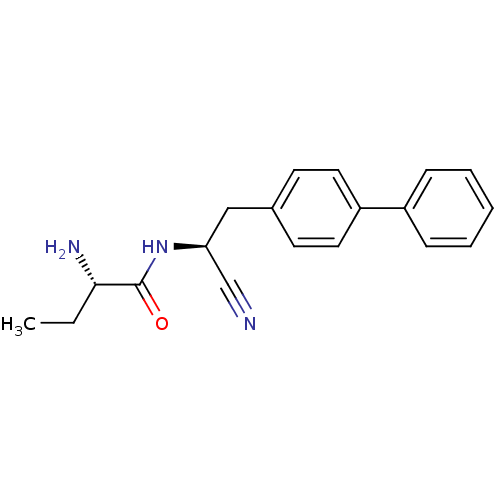 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50186088 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246998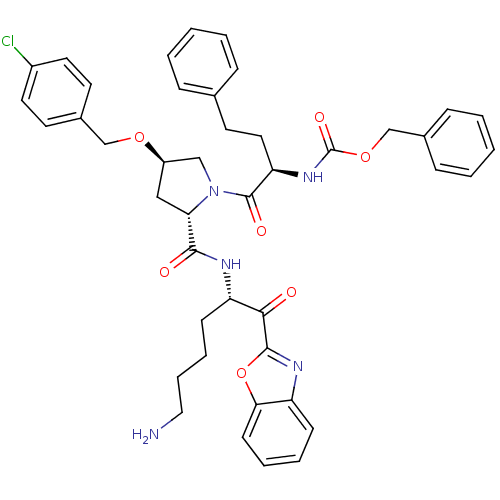 (CHEMBL505558 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246999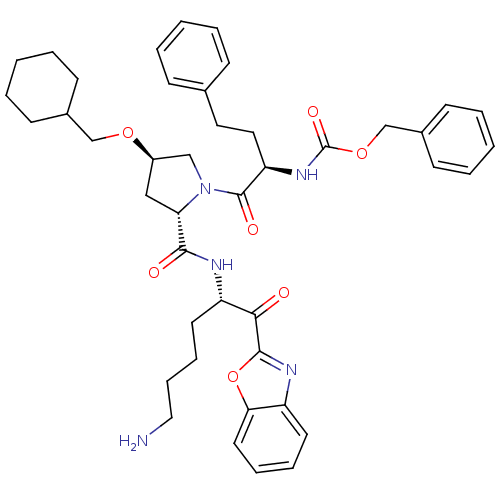 (CHEMBL500474 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246997 (CHEMBL505738 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246995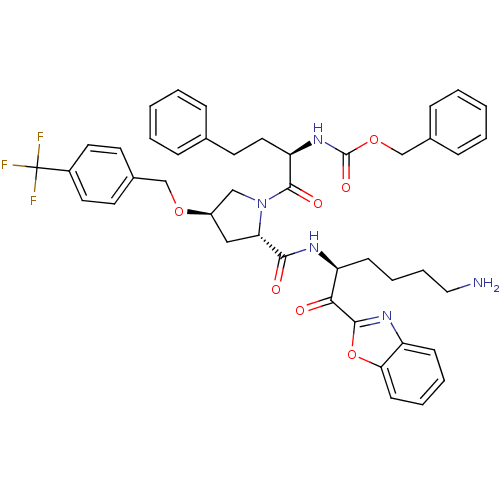 (CHEMBL505048 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247004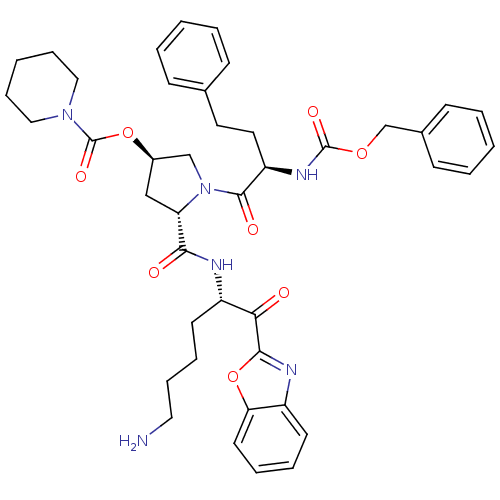 ((3R,5S)-5-(((S)-6-amino-1-(benzo[d]oxazol-2-yl)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246992 (CHEMBL498914 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246994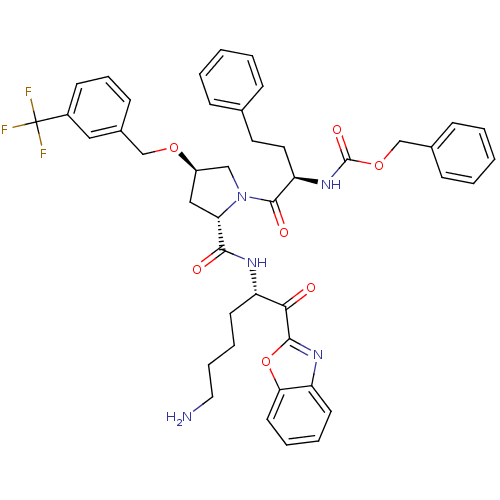 (CHEMBL443101 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246996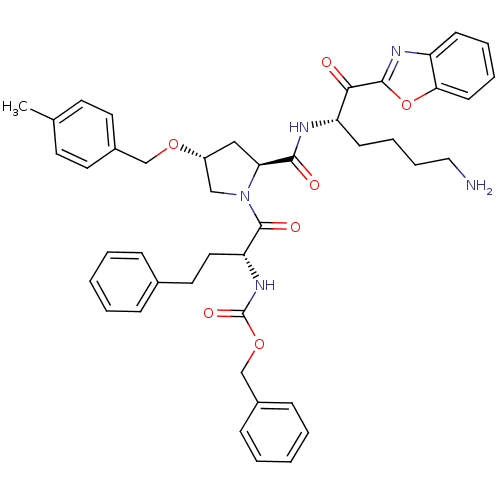 (CHEMBL506226 | {(R)-1-[(2S,4R)-2-[(S)-5-Amino-1-(b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246991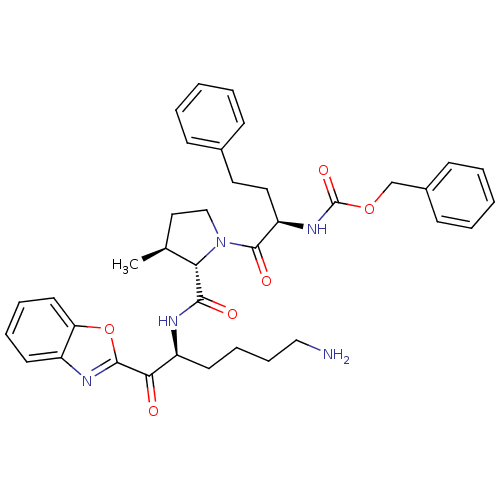 (CHEMBL509770 | benzyl (R)-1-((2S,3S)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247002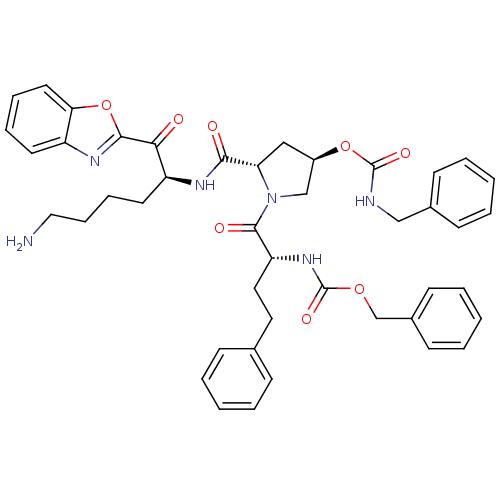 (((R)-1-{(2S,4R)-2-[(S)-5-Amino-1-(benzooxazole-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50243232 (CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247000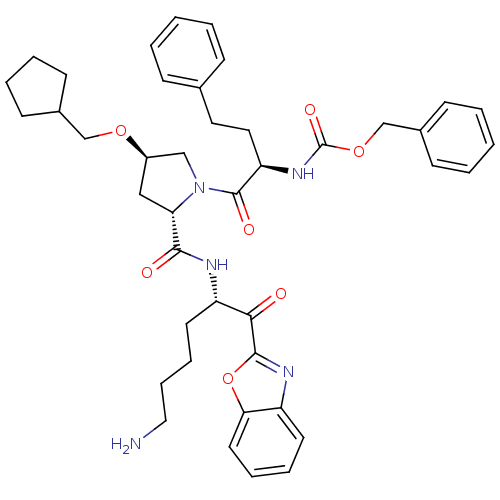 (CHEMBL446259 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247005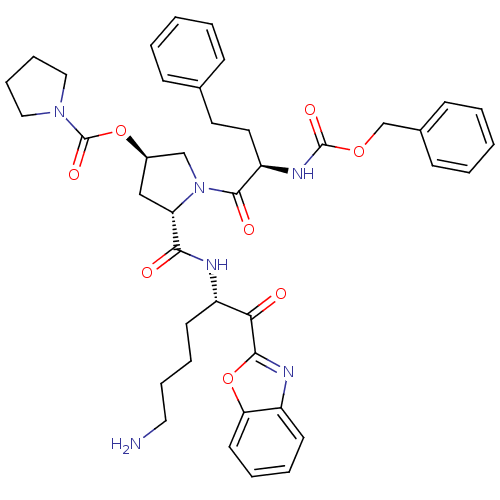 ((3R,5S)-5-(((S)-6-amino-1-(benzo[d]oxazol-2-yl)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50247192 ((S)-2-amino-N-((S)-5-phenyl-1-(phenylsulfonyl)pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247001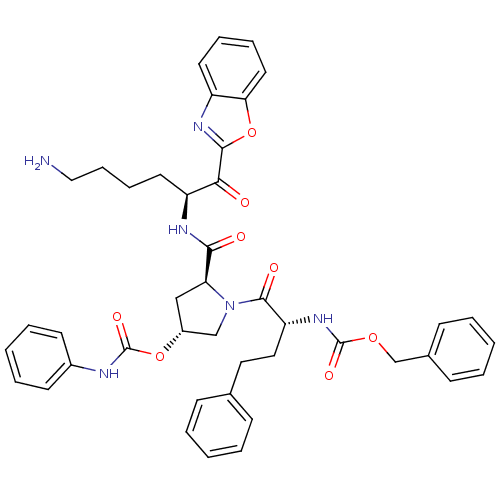 (CHEMBL448873 | Phenyl-carbamic acid (3R,5S)-5-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50247192 ((S)-2-amino-N-((S)-5-phenyl-1-(phenylsulfonyl)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247003 (CHEMBL501945 | {(R)-1-[(2S,4R)-2-[(S)-5-Amino-1-(b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246990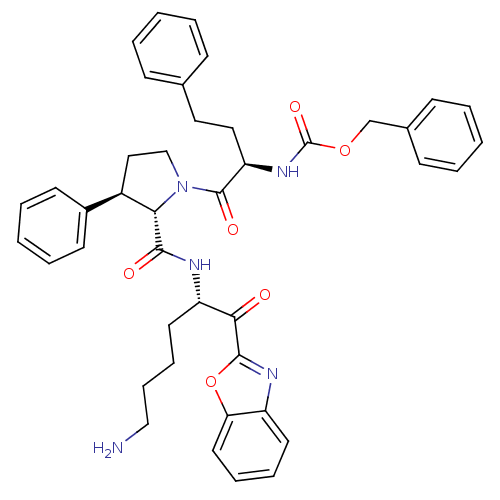 (CHEMBL454436 | benzyl (R)-1-((2S,3R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247006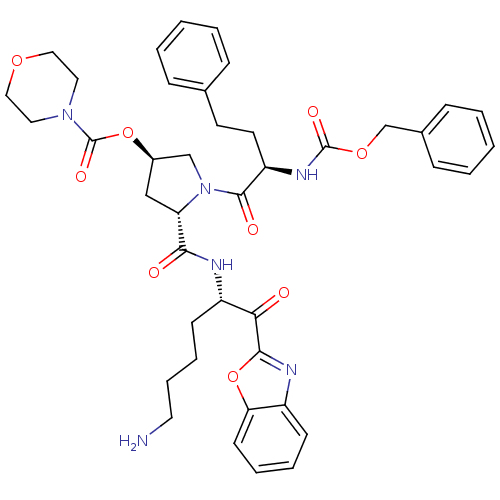 ((3R,5S)-5-((S)-6-amino-1-(benzo[d]oxazol-2-yl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246993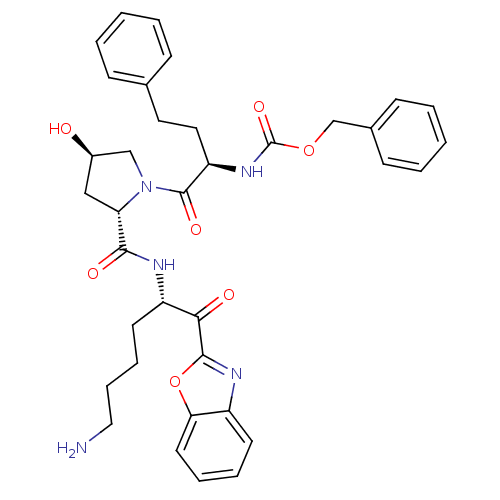 (CHEMBL507245 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50247192 ((S)-2-amino-N-((S)-5-phenyl-1-(phenylsulfonyl)pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50243232 (CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246989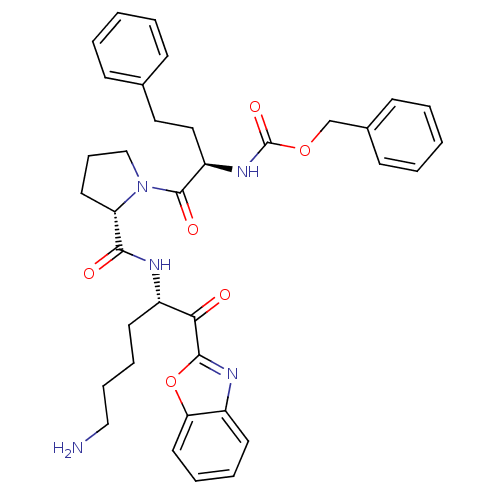 (CHEMBL501802 | benzyl (R)-1-((S)-2-(((S)-6-amino-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247007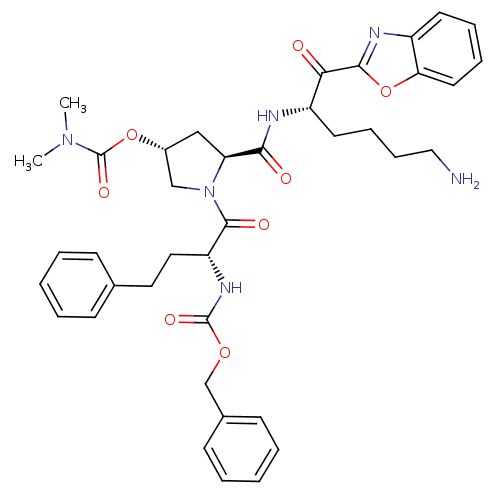 (((R)-1-{(2S,4R)-2-[(S)-5-Amino-1-(benzooxazole-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246988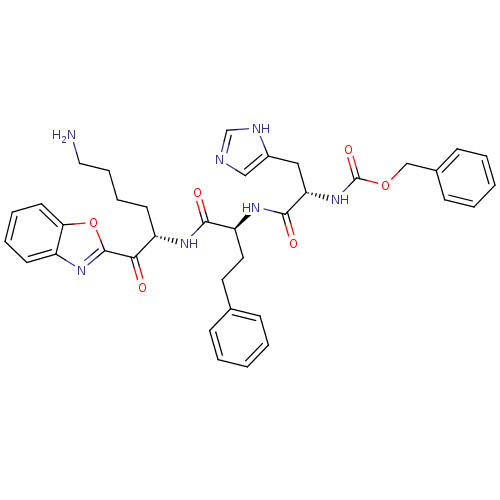 (CHEMBL503925 | benzyl (S)-1-((S)-1-((S)-6-amino-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50186088 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246986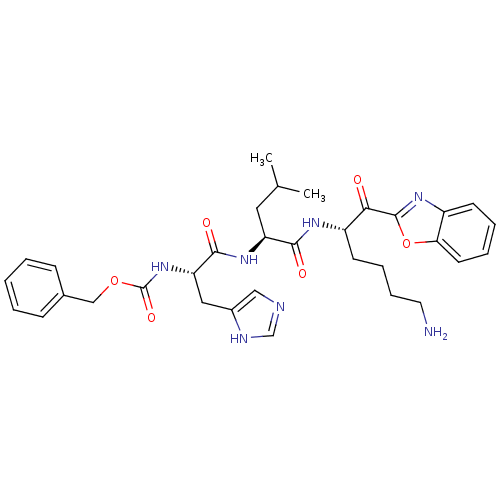 (CHEMBL507205 | benzyl (S)-1-((S)-1-((S)-6-amino-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246987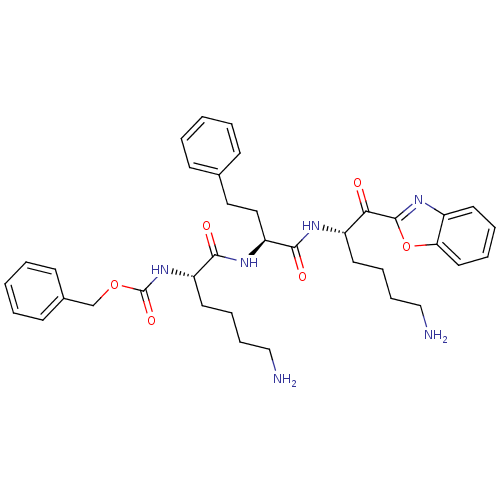 (CHEMBL504394 | benzyl (S)-6-amino-1-((S)-1-((S)-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246985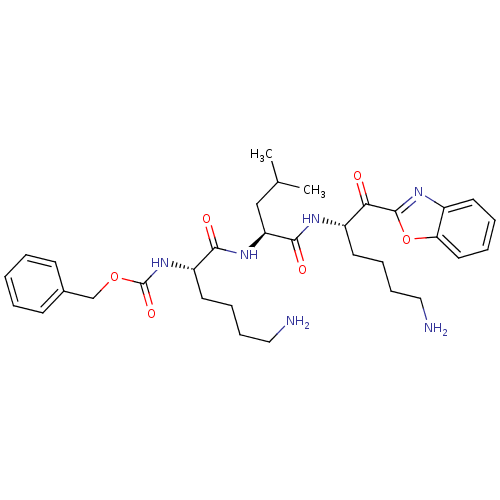 (CHEMBL507704 | benzyl (S)-6-amino-1-((S)-1-((S)-6-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50186088 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246983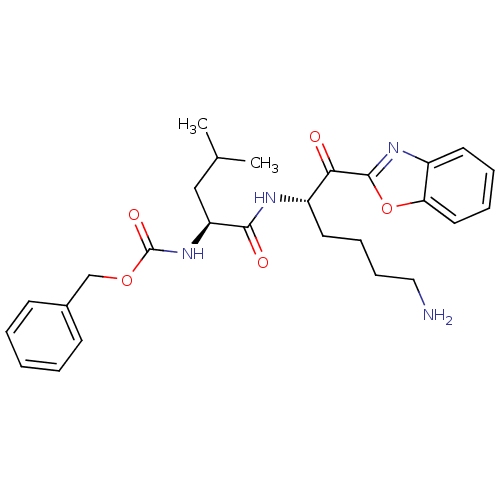 (CHEMBL509923 | benzyl (S)-1-((S)-6-amino-1-(benzo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246984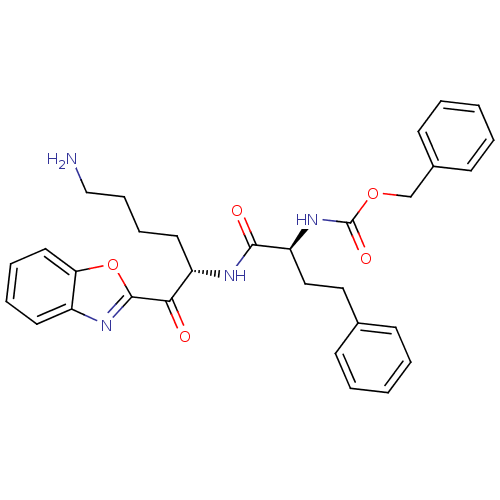 (CHEMBL508055 | benzyl (S)-1-((S)-6-amino-1-(benzo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM294911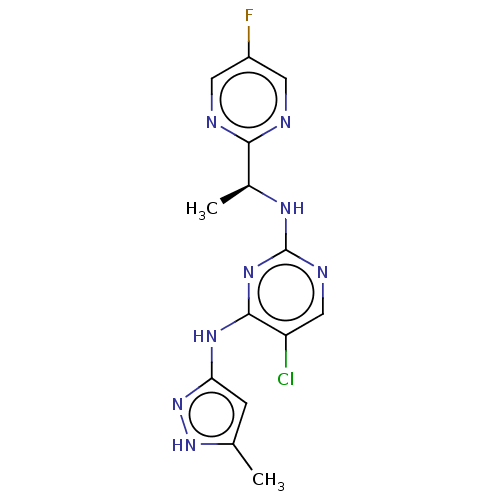 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50021656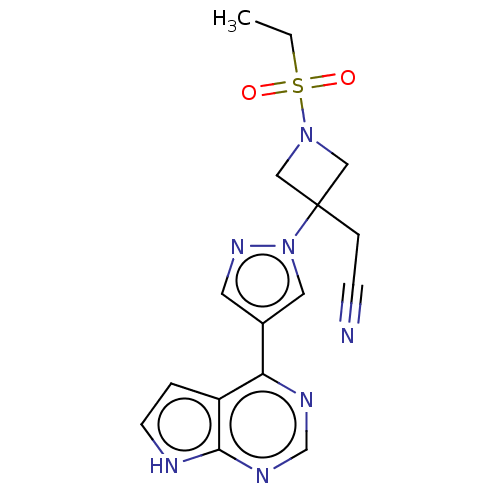 (BARICITINIB | INCB-028050 | LY-3009104 | US1011290...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 [808-1132] (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM50021656 (BARICITINIB | INCB-028050 | LY-3009104 | US1011290...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM50021656 (BARICITINIB | INCB-028050 | LY-3009104 | US1011290...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 [808-1132] (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50402074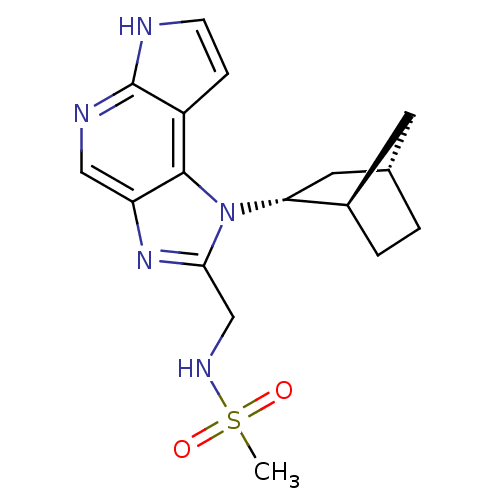 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM294916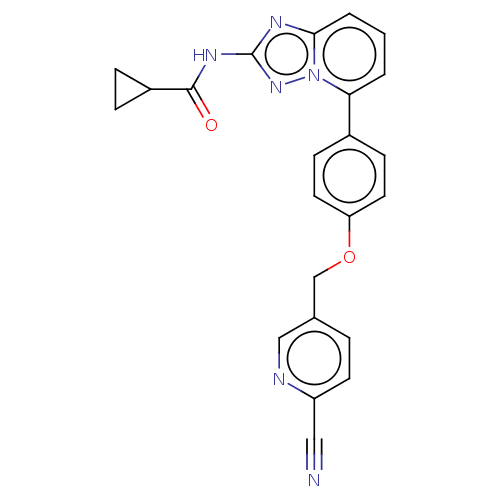 (US10112907, Example 00027 | US10206907, Compound 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM50355501 (INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1218 total ) | Next | Last >> |