

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538098 (CHEMBL4640031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538084 (CHEMBL4647659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human DYRK1A (129 to 509 residues) expressed in mammalian expression system by Kinomescan method | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538099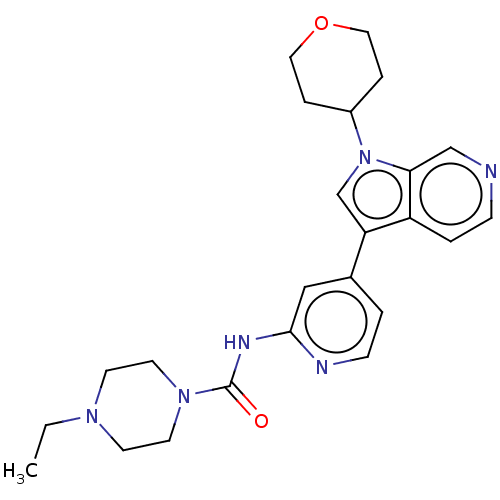 (CHEMBL4636064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538095 (CHEMBL4649473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538090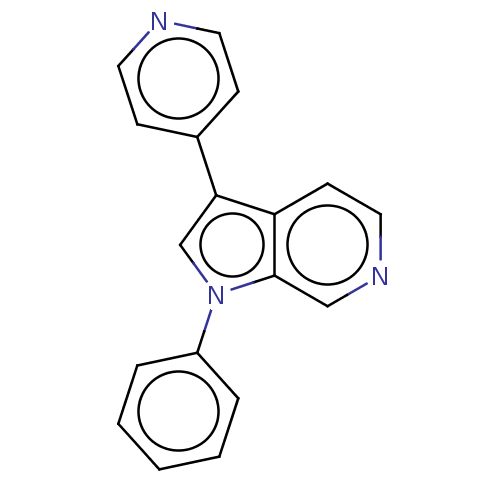 (CHEMBL4648742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252816 (2-(4-{Butyl[3-(2-hydroxyethoxy)benzyl]amino}phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538093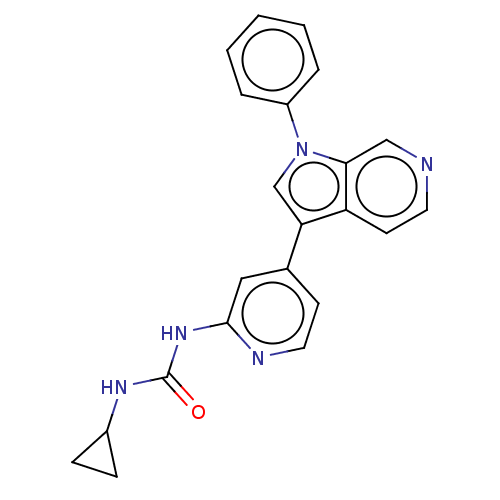 (CHEMBL4645474) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50538084 (CHEMBL4647659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK3beta (1 to 433 residues) expressed in mammalian expression system by Kinomescan method | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538094 (CHEMBL4641631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508126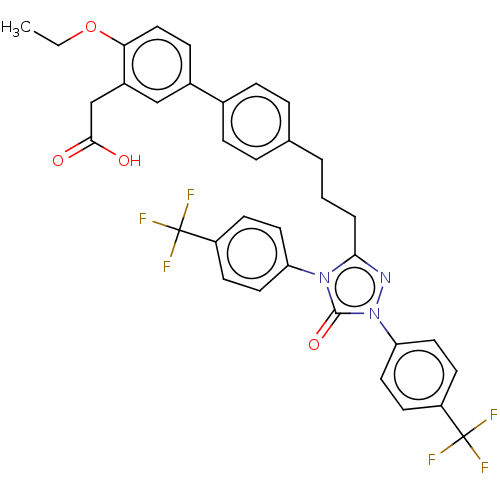 (CHEMBL4457564) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538091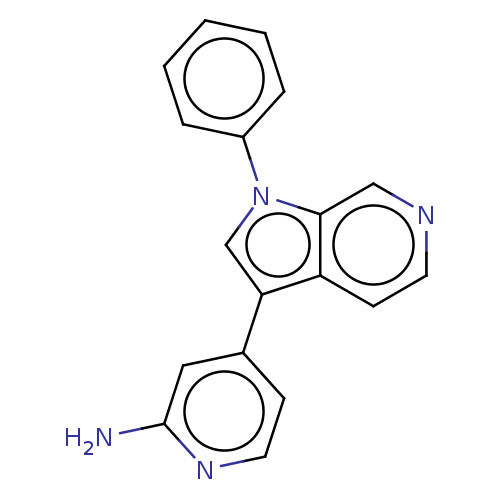 (CHEMBL4640465) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508159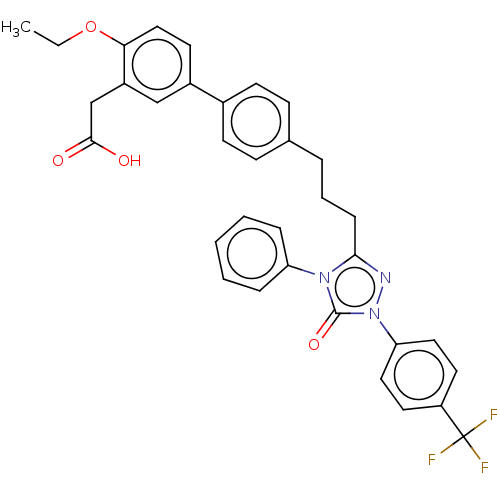 (CHEMBL4529735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508137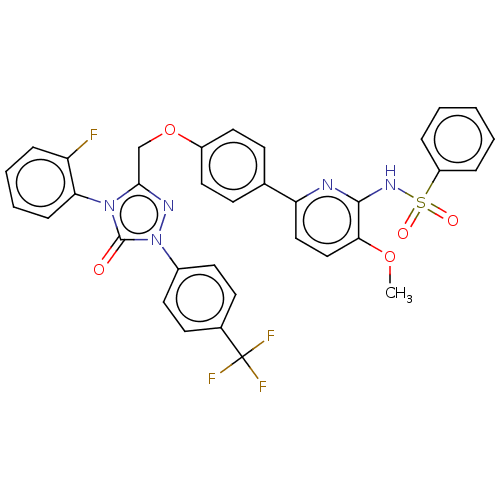 (CHEMBL4437146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508153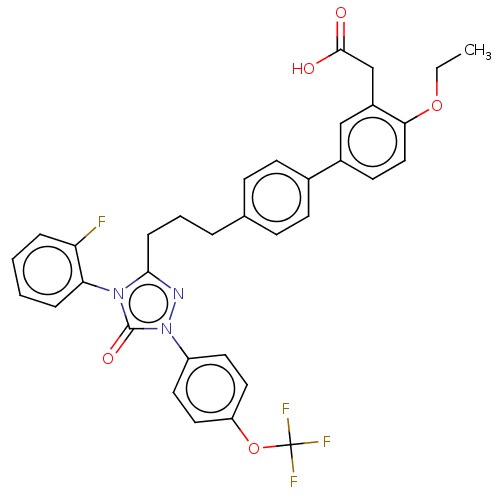 (CHEMBL4549922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508144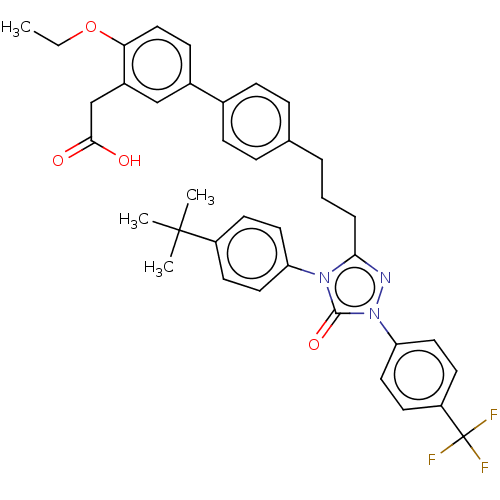 (CHEMBL4545223) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508159 (CHEMBL4529735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252939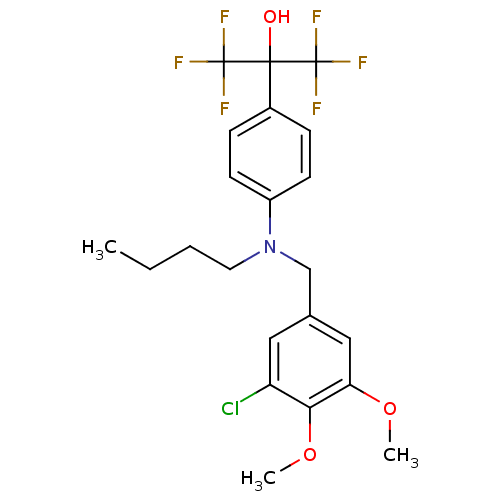 (2-[4-(Butyl{[3-chloro-4,5-bis(methyloxy)phenyl]met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252850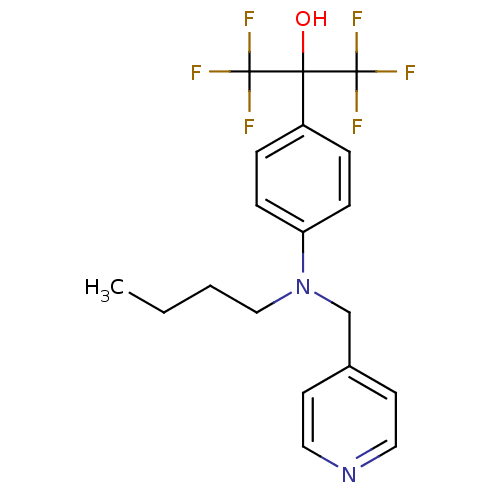 (2-{4-[Butyl(pyridin-4-ylmethyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538096 (CHEMBL4641672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508127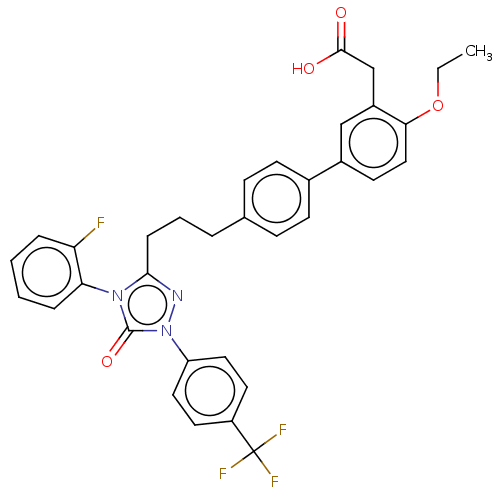 (CHEMBL4449687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252817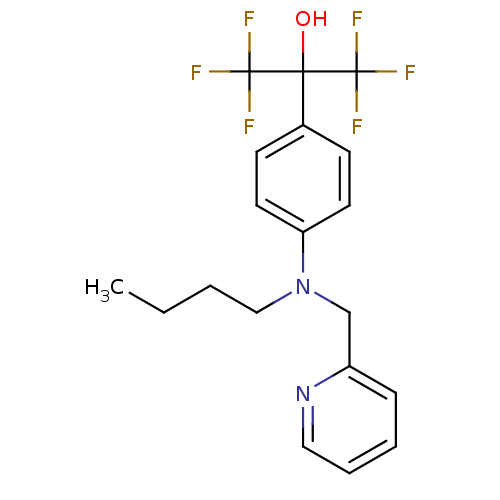 (2-{4-[Butyl(pyridin-2-ylmethyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508145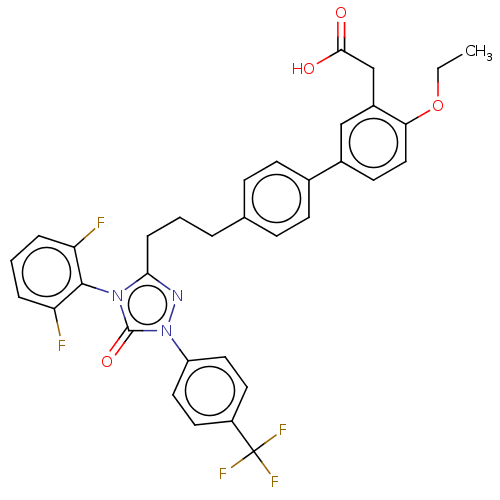 (CHEMBL4450146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252967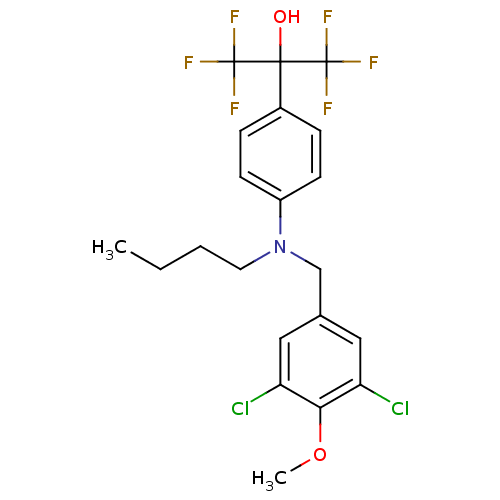 (2-{4-[Butyl(3,5-dichloro-4-methoxybenzyl)amino]phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252941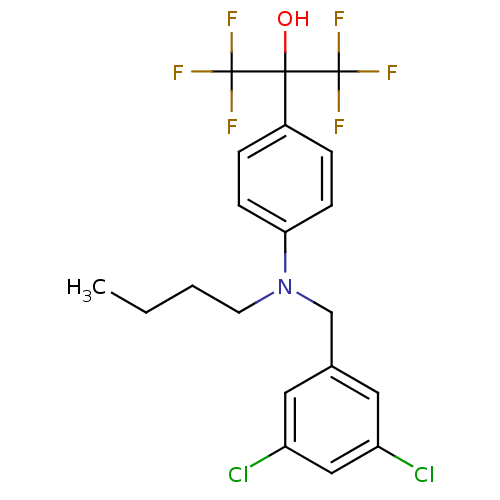 (2-{4-[Butyl(3,5-dichlorobenzyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252852 (3-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252940 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508147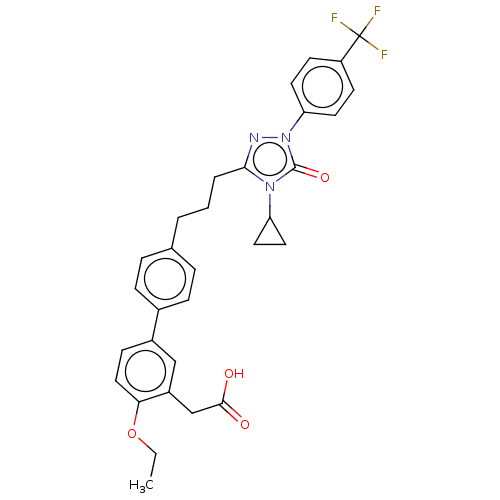 (CHEMBL4530053) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508151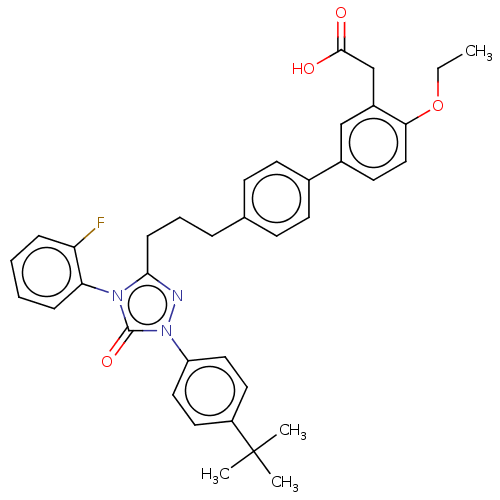 (CHEMBL4449183) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508126 (CHEMBL4457564) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508143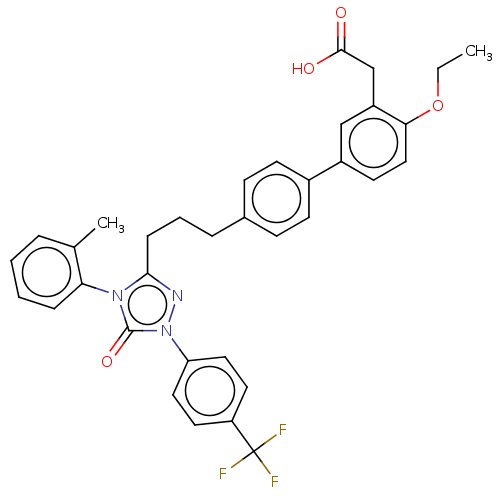 (CHEMBL4443720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50013729 (CHEMBL3264919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at human PPARalpha | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252851 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50538083 (CHEMBL4638292) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK3beta (1 to 433 residues) expressed in mammalian expression system by Kinomescan method | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252815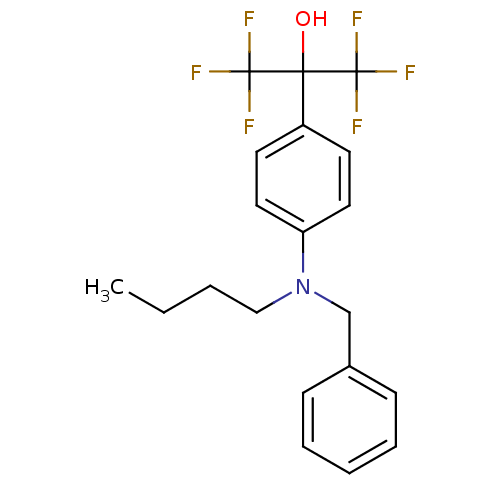 (2-{4-[Benzyl(butyl)amino]phenyl}-1,1,1,3,3,3-hexaf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538085 (CHEMBL4634969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50538097 (CHEMBL4641839) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged DYRK1A (1 to 763 residues) expressed in Sf21 cells using Ulight-glycogen synthase as substrate ... | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252938 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508155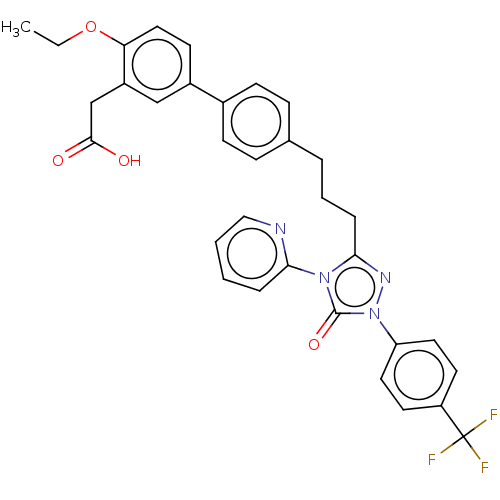 (CHEMBL4592038) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252908 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508125 (CHEMBL4533138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508128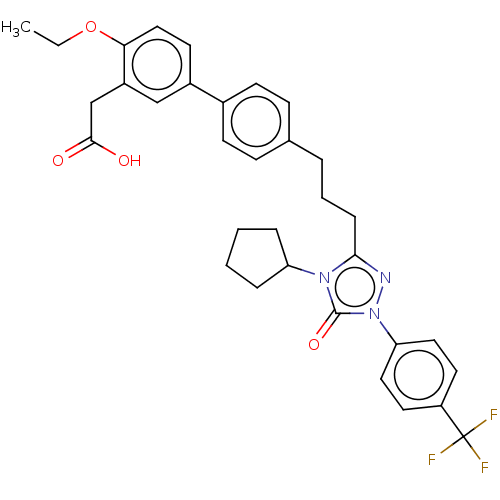 (CHEMBL4539262) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508132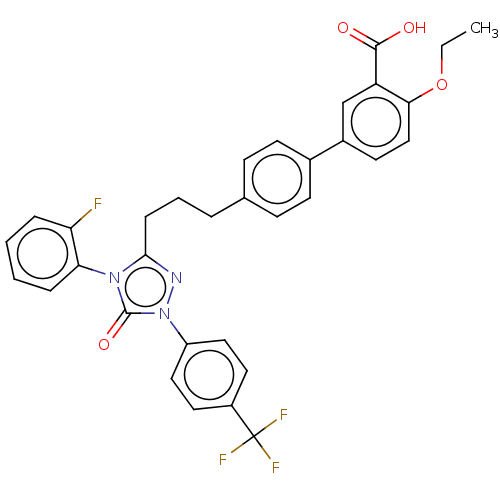 (CHEMBL4546054) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508141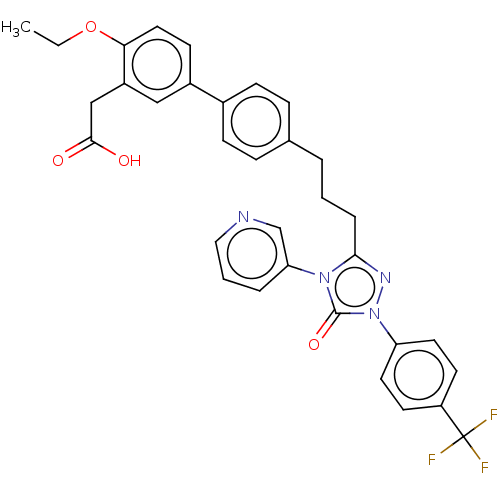 (CHEMBL4447577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508146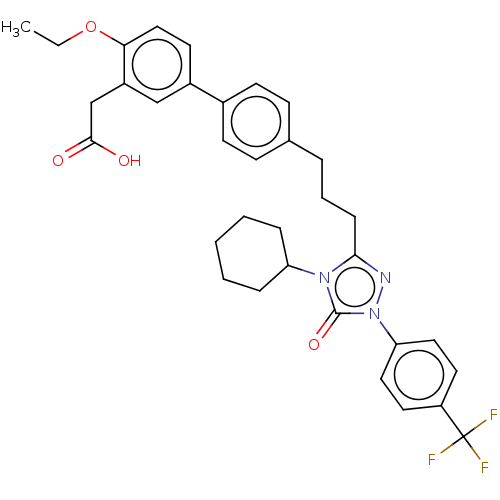 (CHEMBL4587456) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50508146 (CHEMBL4587456) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of DYRK1A (unknown origin) | J Med Chem 63: 2958-2973 (2020) Article DOI: 10.1021/acs.jmedchem.9b01624 BindingDB Entry DOI: 10.7270/Q2WQ079V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50508150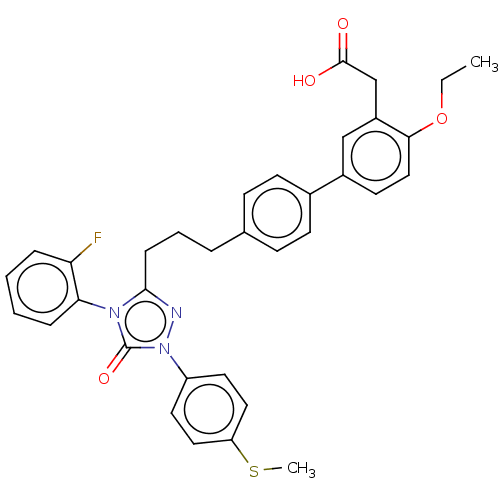 (CHEMBL4547480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences Curated by ChEMBL | Assay Description Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba... | Bioorg Med Chem Lett 29: 503-508 (2019) Article DOI: 10.1016/j.bmcl.2018.12.045 BindingDB Entry DOI: 10.7270/Q2XK8JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 326 total ) | Next | Last >> |