| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50104235 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_39629 |
|---|
| IC50 | 7±n/a nM |
|---|
| Citation |  Hale, JJ; Budhu, RJ; Holson, EB; Finke, PE; Oates, B; Mills, SG; MacCoss, M; Gould, SL; DeMartino, JA; Springer, MS; Siciliano, S; Malkowitz, L; Schleif, WA; Hazuda, D; Miller, M; Kessler, J; Danzeisen, R; Holmes, K; Lineberger, J; Carella, A; Carver, G; Emini, E 1,3,4-Trisubstituted pyrrolidine CCR5 receptor antagonists. Part 2: lead optimization affording selective, orally bioavailable compounds with potent anti-HIV activity. Bioorg Med Chem Lett11:2741-5 (2001) [PubMed] Hale, JJ; Budhu, RJ; Holson, EB; Finke, PE; Oates, B; Mills, SG; MacCoss, M; Gould, SL; DeMartino, JA; Springer, MS; Siciliano, S; Malkowitz, L; Schleif, WA; Hazuda, D; Miller, M; Kessler, J; Danzeisen, R; Holmes, K; Lineberger, J; Carella, A; Carver, G; Emini, E 1,3,4-Trisubstituted pyrrolidine CCR5 receptor antagonists. Part 2: lead optimization affording selective, orally bioavailable compounds with potent anti-HIV activity. Bioorg Med Chem Lett11:2741-5 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50104235 |
|---|
| n/a |
|---|
| Name | BDBM50104235 |
|---|
| Synonyms: | CHEMBL85086 | {1-[(S)-4-(Benzenesulfonyl-methyl-amino)-3-phenyl-butyl]-piperidin-4-yl}-ethyl-carbamic acid benzyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H41N3O4S |
|---|
| Mol. Mass. | 563.751 |
|---|
| SMILES | CCN(C1CCN(CC[C@H](CN(C)S(=O)(=O)c2ccccc2)c2ccccc2)CC1)C(=O)OCc1ccccc1 |
|---|
| Structure | 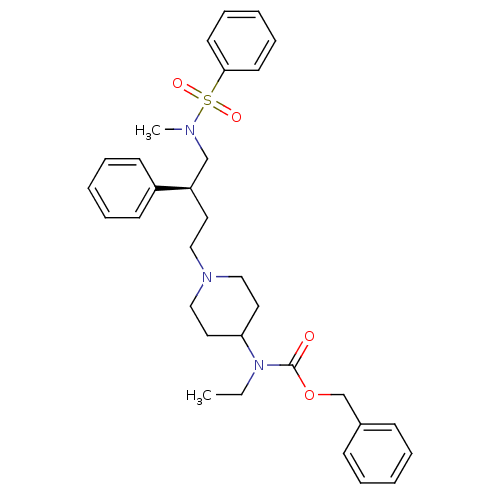
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hale, JJ; Budhu, RJ; Holson, EB; Finke, PE; Oates, B; Mills, SG; MacCoss, M; Gould, SL; DeMartino, JA; Springer, MS; Siciliano, S; Malkowitz, L; Schleif, WA; Hazuda, D; Miller, M; Kessler, J; Danzeisen, R; Holmes, K; Lineberger, J; Carella, A; Carver, G; Emini, E 1,3,4-Trisubstituted pyrrolidine CCR5 receptor antagonists. Part 2: lead optimization affording selective, orally bioavailable compounds with potent anti-HIV activity. Bioorg Med Chem Lett11:2741-5 (2001) [PubMed]
Hale, JJ; Budhu, RJ; Holson, EB; Finke, PE; Oates, B; Mills, SG; MacCoss, M; Gould, SL; DeMartino, JA; Springer, MS; Siciliano, S; Malkowitz, L; Schleif, WA; Hazuda, D; Miller, M; Kessler, J; Danzeisen, R; Holmes, K; Lineberger, J; Carella, A; Carver, G; Emini, E 1,3,4-Trisubstituted pyrrolidine CCR5 receptor antagonists. Part 2: lead optimization affording selective, orally bioavailable compounds with potent anti-HIV activity. Bioorg Med Chem Lett11:2741-5 (2001) [PubMed]