

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50071484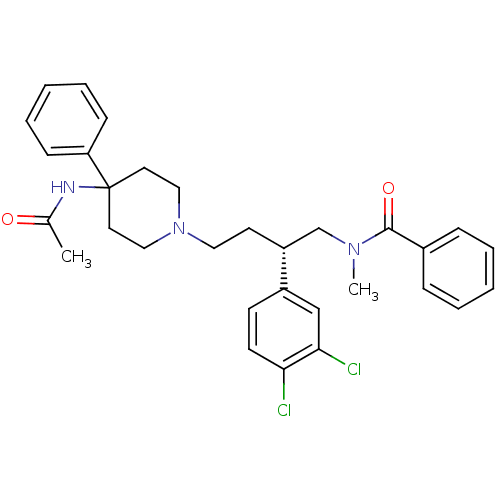 (CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro to inhibit the binding of [125I]-NKA to its receptor in rat duodenum membrane | Bioorg Med Chem Lett 3: 319-322 (1993) Article DOI: 10.1016/S0960-894X(01)80901-X BindingDB Entry DOI: 10.7270/Q2CR5T9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM235953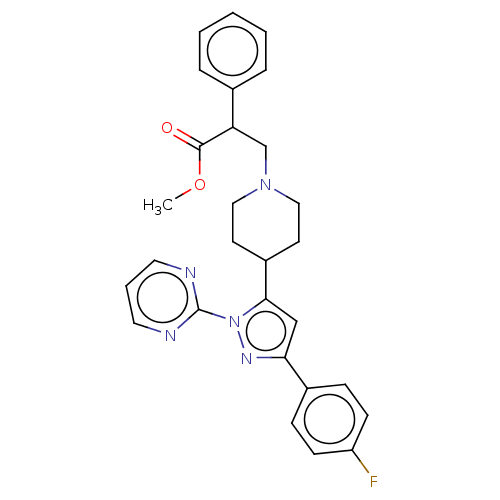 (US9365539, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The assay was run in a 384-well microliter plate at 37° C. with a total volume of 50 uL. Final assay concentrations were 0.13 nM human PrCP (CHO) or ... | US Patent US9365539 (2016) BindingDB Entry DOI: 10.7270/Q22B8WXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50364407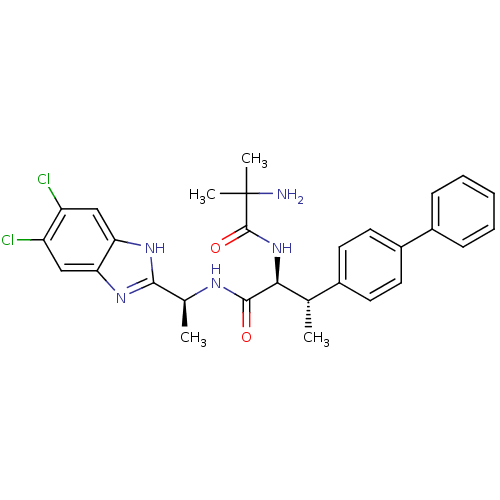 (CHEMBL1950444) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 22: 1774-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.064 BindingDB Entry DOI: 10.7270/Q27H1K28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50158348 ((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 3351-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.106 BindingDB Entry DOI: 10.7270/Q2QN667K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM235950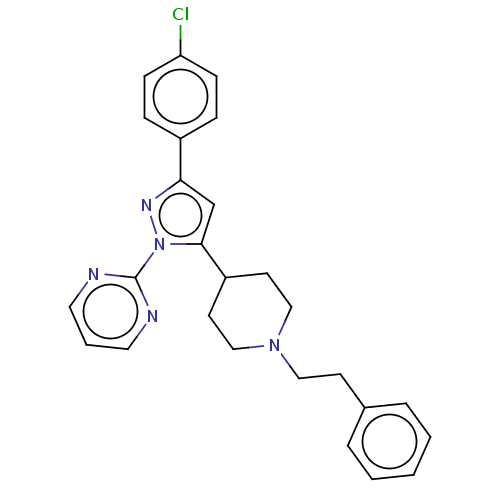 (US9365539, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The assay was run in a 384-well microliter plate at 37° C. with a total volume of 50 uL. Final assay concentrations were 0.13 nM human PrCP (CHO) or ... | US Patent US9365539 (2016) BindingDB Entry DOI: 10.7270/Q22B8WXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121838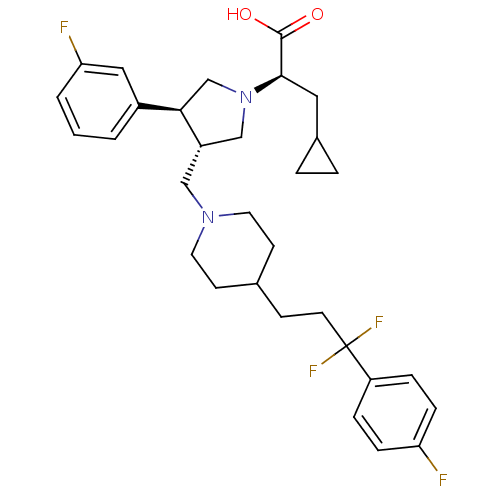 ((R)-3-cyclopropyl-2-((3S,4S)-3-((4-(3,3-difluoro-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067933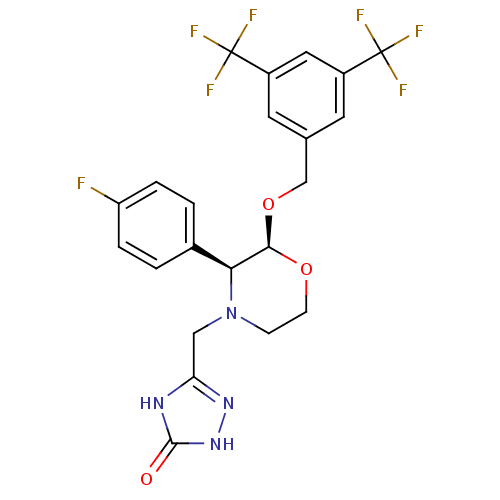 (5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50443348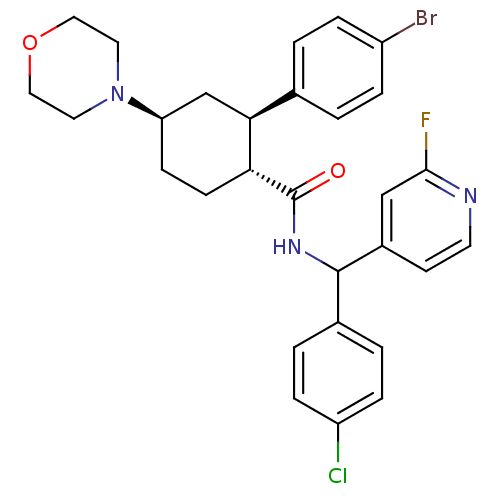 (CHEMBL3086040 | US8669252, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay | Bioorg Med Chem Lett 23: 6228-33 (2013) Article DOI: 10.1016/j.bmcl.2013.09.094 BindingDB Entry DOI: 10.7270/Q2DZ09Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50408664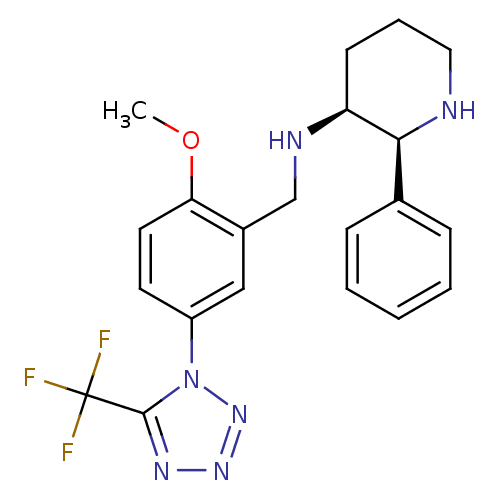 (GR-205171 | VOFOPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50049469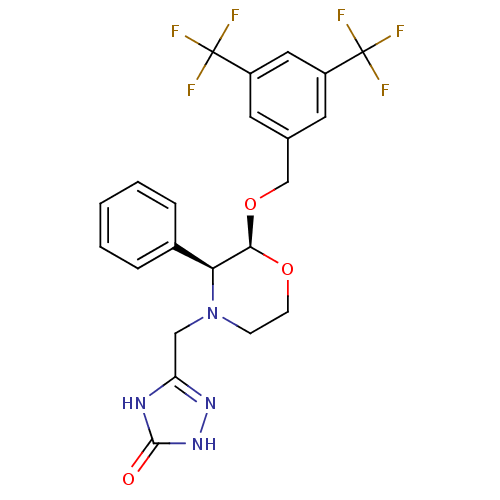 (5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 39: 1760-2 (1996) Article DOI: 10.1021/jm950654w BindingDB Entry DOI: 10.7270/Q2GB2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136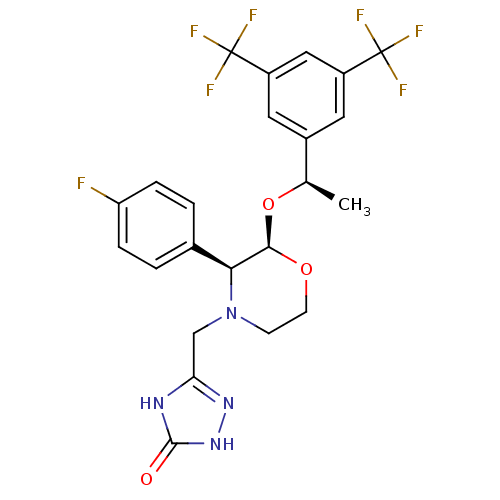 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50220136 (3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compounds were evaluated for inhibitory activity against human Tachykinin receptor 1 | J Med Chem 43: 1234-41 (2000) BindingDB Entry DOI: 10.7270/Q2G73CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50049469 (5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067940 (5-{(2R,3S)-2-[(S)-1-(3,5-Bis-trifluoromethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067939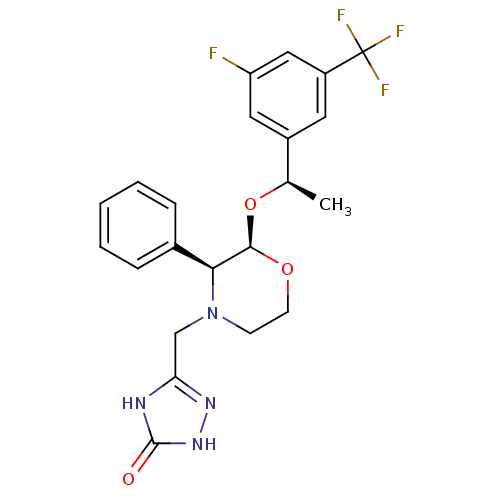 (5-{(2R,3S)-2-[(R)-1-(3-Fluoro-5-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121836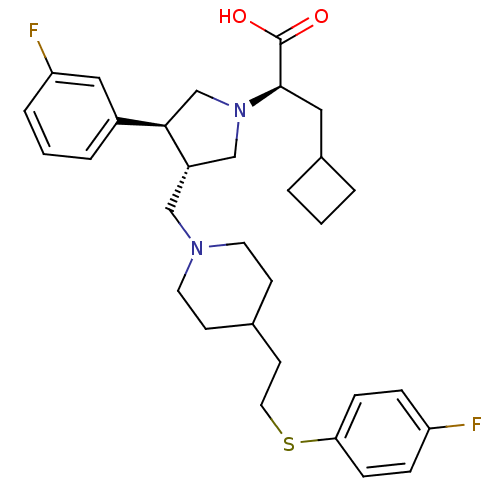 ((R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338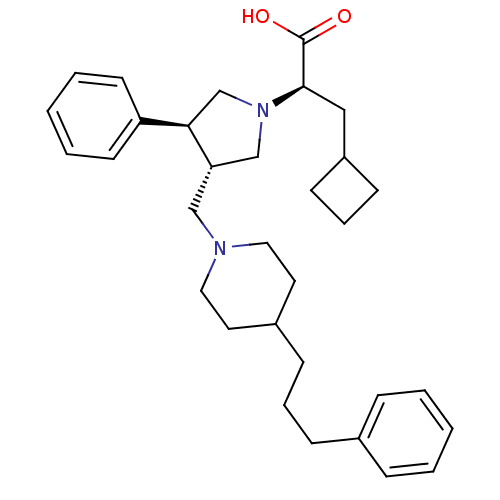 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119349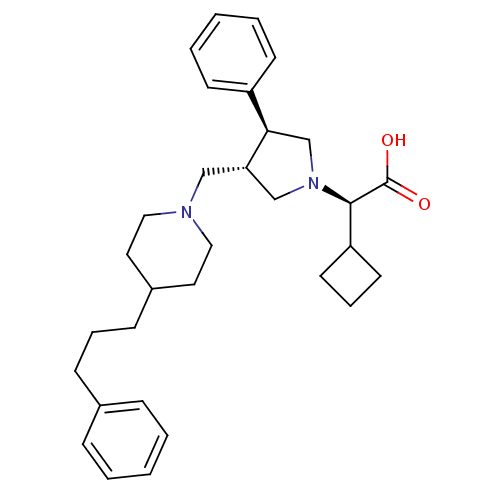 ((R)-2-cyclobutyl-2-((3S,4S)-3-phenyl-4-((4-(3-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119341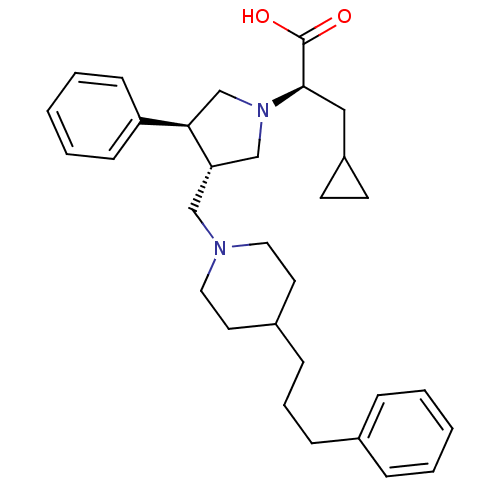 ((R)-3-cyclopropyl-2-((3S,4S)-3-phenyl-4-((4-(3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50147703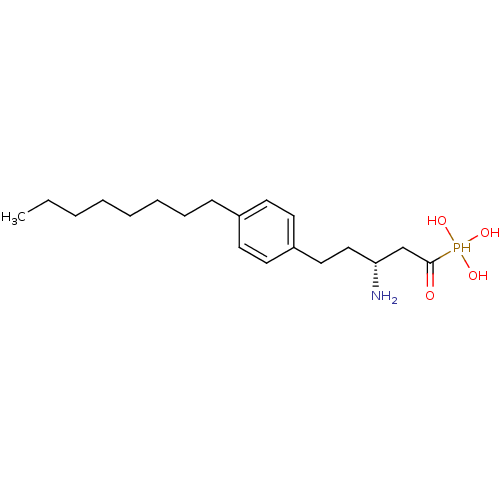 (CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 3351-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.106 BindingDB Entry DOI: 10.7270/Q2QN667K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50147703 (CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand | Bioorg Med Chem Lett 14: 3495-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.069 BindingDB Entry DOI: 10.7270/Q2SF2WRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119336 (2-cyclohexyl-2-{3-phenyl-4-[4-(3-phenylpropyl)hexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105517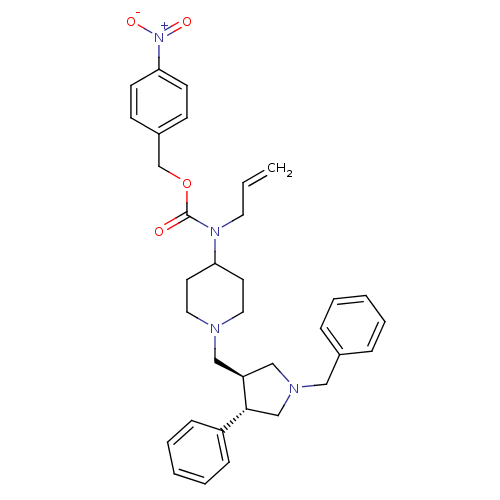 (Allyl-[1-((3S,4S)-1-benzyl-4-phenyl-pyrrolidin-3-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105510 (Allyl-{1-[(S)-4-(benzenesulfonyl-methyl-amino)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50364689 (CHEMBL1951478) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse PrCP | Bioorg Med Chem Lett 22: 1550-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.002 BindingDB Entry DOI: 10.7270/Q2JS9QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50110088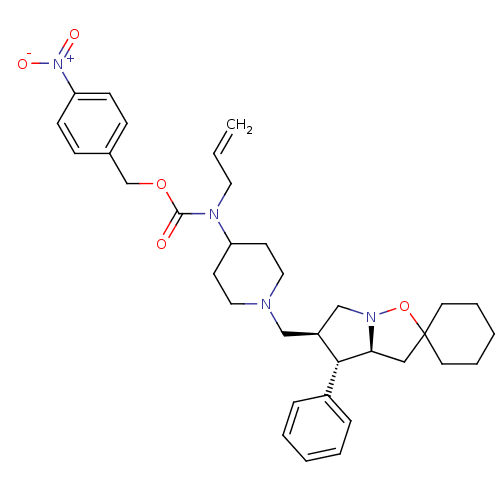 (4N-allyl-4N-[4-nitrobenzyloxycarboyl]-1-[4'-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 12: 677-9 (2002) BindingDB Entry DOI: 10.7270/Q2DF6QHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50049467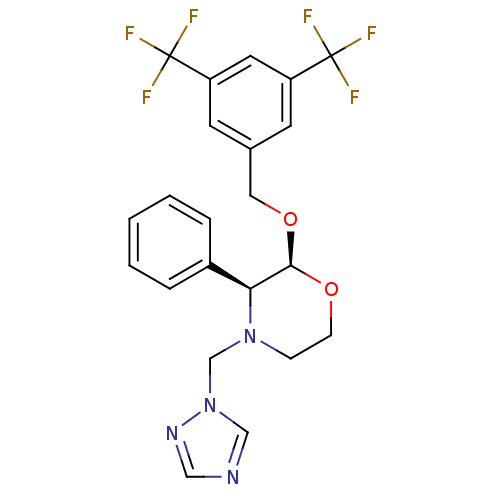 ((2S,3S)-2-(3,5-Bis-trifluoromethyl-benzyloxy)-3-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 39: 1760-2 (1996) Article DOI: 10.1021/jm950654w BindingDB Entry DOI: 10.7270/Q2GB2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067935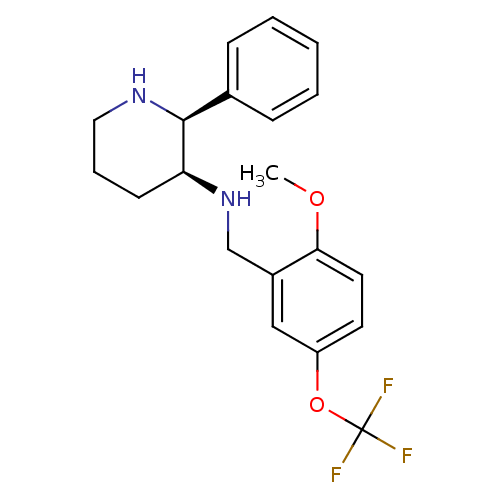 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells | J Med Chem 41: 4607-14 (1998) Article DOI: 10.1021/jm980299k BindingDB Entry DOI: 10.7270/Q2XW4HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152328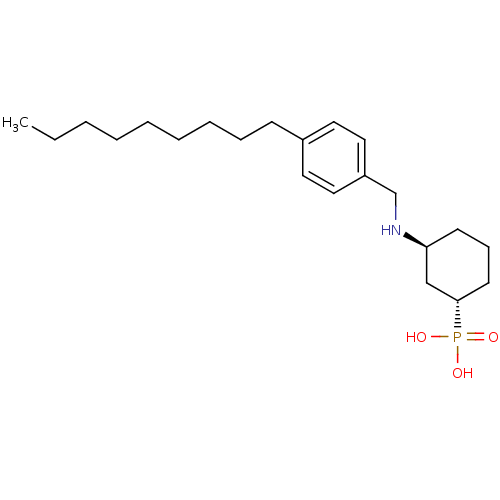 (CHEMBL181597 | [(1S,3S)-3-(4-Nonyl-benzylamino)-cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50158348 ((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 3351-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.106 BindingDB Entry DOI: 10.7270/Q2QN667K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152322 (CHEMBL184879 | {2-[5-(4-Nonyl-phenyl)-pyrrolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50147713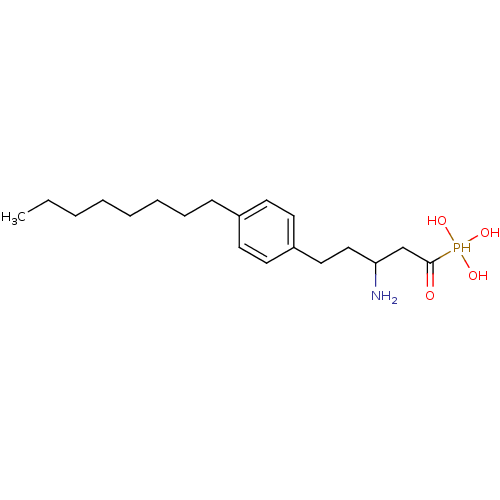 (CHEMBL113344 | [3-Amino-1-hydroxy-5-(4-octyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 3351-5 (2004) Article DOI: 10.1016/j.bmcl.2004.02.106 BindingDB Entry DOI: 10.7270/Q2QN667K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50152322 (CHEMBL184879 | {2-[5-(4-Nonyl-phenyl)-pyrrolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes | Bioorg Med Chem Lett 14: 4861-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.049 BindingDB Entry DOI: 10.7270/Q2BK1D38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364386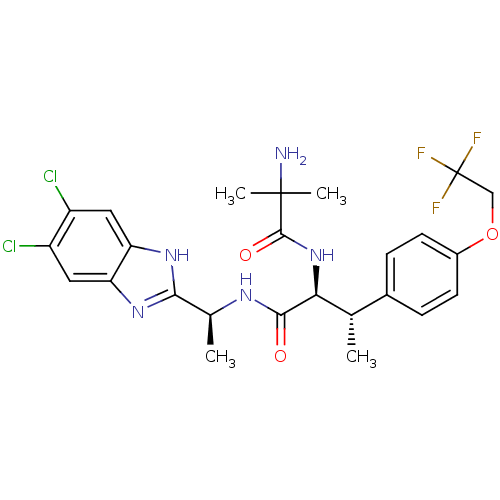 (CHEMBL1950440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay | Bioorg Med Chem Lett 22: 1774-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.064 BindingDB Entry DOI: 10.7270/Q27H1K28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50443351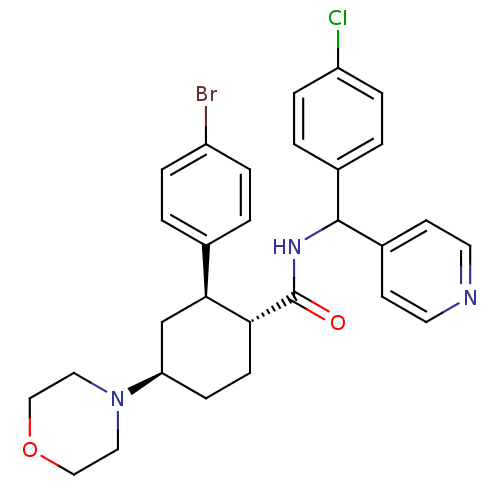 (CHEMBL3086037 | US8669252, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay | Bioorg Med Chem Lett 23: 6228-33 (2013) Article DOI: 10.1016/j.bmcl.2013.09.094 BindingDB Entry DOI: 10.7270/Q2DZ09Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50364676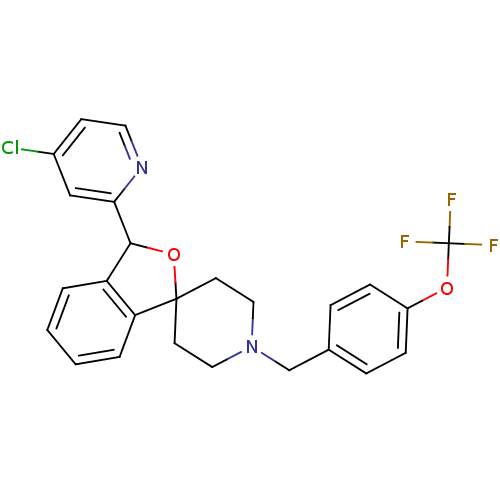 (CHEMBL1951465 | US8785634, 1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse PrCP | Bioorg Med Chem Lett 22: 1550-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.002 BindingDB Entry DOI: 10.7270/Q2JS9QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50148394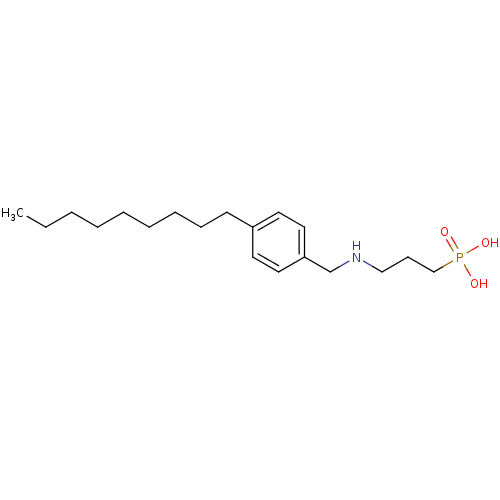 (CHEMBL118860 | [3-(4-Nonyl-benzylamino)-propyl]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand | Bioorg Med Chem Lett 14: 3495-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.069 BindingDB Entry DOI: 10.7270/Q2SF2WRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119342 ((R)-2-cyclopentyl-2-((3S,4S)-3-phenyl-4-((4-(3-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119321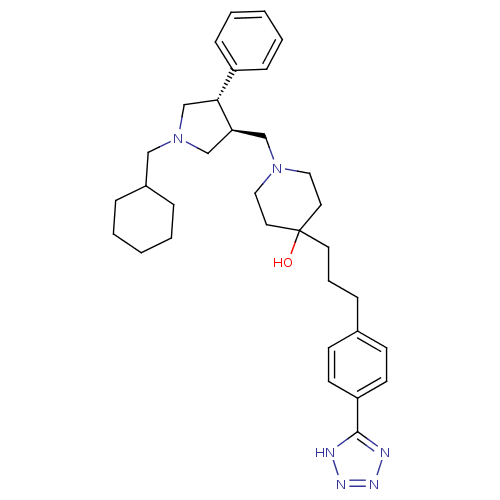 (1-(1-Cyclohexylmethyl-4-phenyl-pyrrolidin-3-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha. | Bioorg Med Chem Lett 12: 3001-4 (2002) BindingDB Entry DOI: 10.7270/Q2MK6C71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50148394 (CHEMBL118860 | [3-(4-Nonyl-benzylamino)-propyl]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human sphingosine 1-phosphate receptor 1 expressed in CHO cells was determined by using [33P]-S1P as radioligand | Bioorg Med Chem Lett 14: 3501-5 (2004) Article DOI: 10.1016/j.bmcl.2004.04.070 BindingDB Entry DOI: 10.7270/Q2W66K76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121828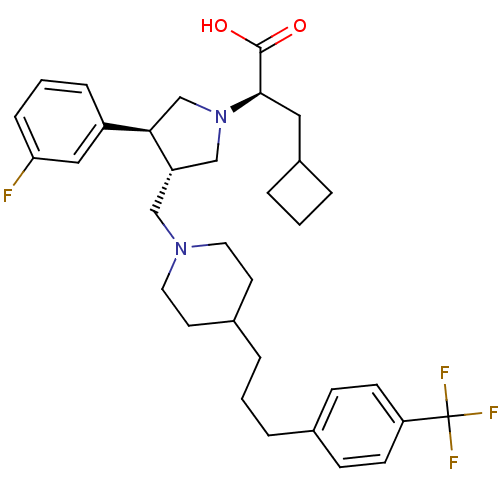 ((R)-3-Cyclobutyl-2-((S)-3-(3-fluoro-phenyl)-4-{4-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121833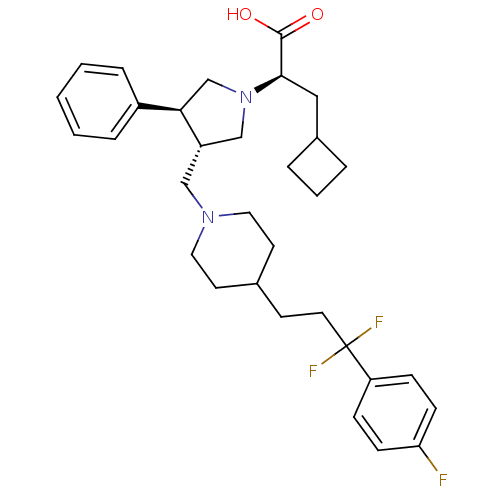 ((R)-3-cyclobutyl-2-((3S,4S)-3-((4-(3,3-difluoro-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119316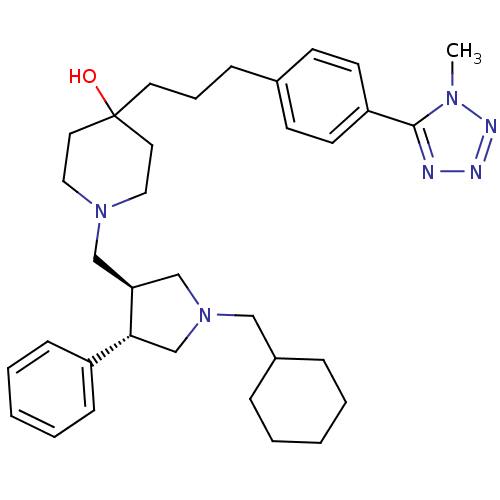 (1-(1-Cyclohexylmethyl-4-phenyl-pyrrolidin-3-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 12: 2997-3000 (2002) BindingDB Entry DOI: 10.7270/Q2RB73ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105503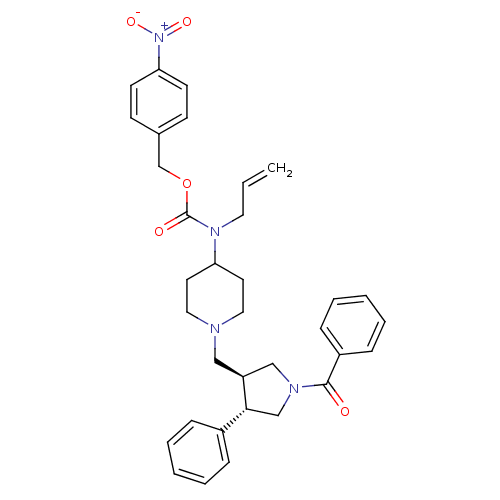 (Allyl-[1-((3S,4S)-1-benzoyl-4-phenyl-pyrrolidin-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50105521 (Allyl-[1-((3S,4S)-1-cyclopentanecarbonyl-4-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cells | Bioorg Med Chem Lett 11: 2741-5 (2001) BindingDB Entry DOI: 10.7270/Q2F47NFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50320187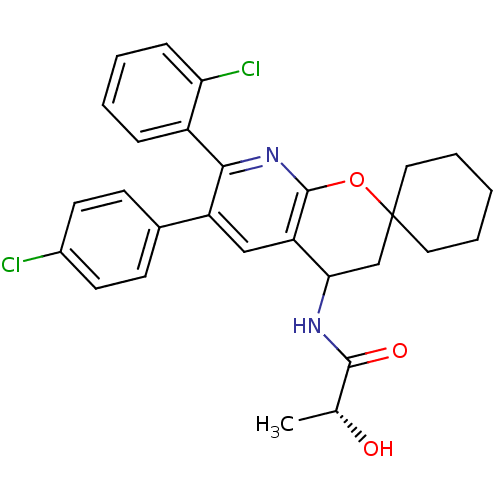 ((2R)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human CB1 receptor | Bioorg Med Chem Lett 20: 3750-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.071 BindingDB Entry DOI: 10.7270/Q22F7NNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2997 total ) | Next | Last >> |