

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Insulin-like growth factor 1 receptor | ||
| Ligand | BDBM50336326 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_716595 (CHEMBL1670374) | ||
| IC50 | 20±n/a nM | ||
| Citation |  Jin, M; Kleinberg, A; Cooke, A; Gokhale, PC; Foreman, K; Dong, H; Siu, KW; Bittner, MA; Mulvihill, KM; Yao, Y; Landfair, D; O'Connor, M; Mak, G; Pachter, JA; Wild, R; Rosenfeld-Franklin, M; Ji, Q; Mulvihill, MJ Potent and selective cyclohexyl-derived imidazopyrazine insulin-like growth factor 1 receptor inhibitors with in vivo efficacy. Bioorg Med Chem Lett21:1176-80 (2011) [PubMed] Article Jin, M; Kleinberg, A; Cooke, A; Gokhale, PC; Foreman, K; Dong, H; Siu, KW; Bittner, MA; Mulvihill, KM; Yao, Y; Landfair, D; O'Connor, M; Mak, G; Pachter, JA; Wild, R; Rosenfeld-Franklin, M; Ji, Q; Mulvihill, MJ Potent and selective cyclohexyl-derived imidazopyrazine insulin-like growth factor 1 receptor inhibitors with in vivo efficacy. Bioorg Med Chem Lett21:1176-80 (2011) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Insulin-like growth factor 1 receptor | |||
| Name: | Insulin-like growth factor 1 receptor | ||
| Synonyms: | CD_antigen=CD221 | IGF-I receptor | IGF1R | IGF1R_HUMAN | Insulin-like growth factor 1 receptor (IGF1R) | Insulin-like growth factor 1 receptor (IGFIR) | Insulin-like growth factor 1 receptor alpha chain | Insulin-like growth factor 1 receptor beta chain | Insulin-like growth factor I receptor | Insulin-like growth factor receptor (IGFR) | ||
| Type: | Protein | ||
| Mol. Mass.: | 154776.79 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P08069 | ||
| Residue: | 1367 | ||
| Sequence: |
| ||
| BDBM50336326 | |||
| n/a | |||
| Name | BDBM50336326 | ||
| Synonyms: | CHEMBL1667944 | trans-4-(8-amino-1-(8-fluoro-2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl)-N-(1H-1,2,4-triazol-5-yl)cyclohexanecarboxamide | ||
| Type | Small organic molecule | ||
| Emp. Form. | C30H26FN9O | ||
| Mol. Mass. | 547.5855 | ||
| SMILES | Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nnc[nH]1 |r,wU:27.31,wD:30.38,(18.99,2.16,;19,.62,;17.67,-.15,;17.67,-1.69,;19,-2.46,;20.34,-1.68,;21.81,-2.15,;22.71,-.9,;21.8,.34,;22.57,1.67,;21.8,3,;22.56,4.33,;24.11,4.33,;24.87,5.66,;26.4,5.66,;27.18,4.33,;26.41,3,;24.88,3,;24.11,1.67,;24.88,.33,;28.71,4.33,;29.48,2.99,;31.02,2.99,;31.79,4.33,;31.01,5.66,;29.47,5.66,;20.33,-.14,;22.3,-3.61,;23.8,-3.91,;24.28,-5.38,;23.25,-6.53,;21.75,-6.21,;21.27,-4.76,;23.73,-7.99,;22.95,-9.32,;25.27,-8.01,;26.03,-9.35,;25.56,-10.81,;26.81,-11.72,;28.05,-10.81,;27.58,-9.35,)| | ||
| Structure | 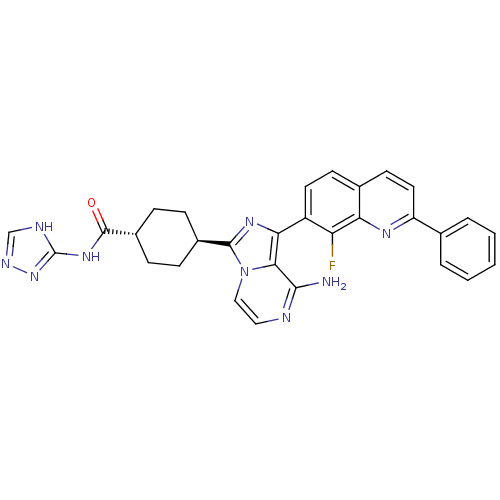
| ||