 Found 185 hits with Last Name = 'pachter' and Initial = 'ja'
Found 185 hits with Last Name = 'pachter' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287720

(6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...)Show SMILES CN1C[C@@H](NC[C@H]1c1c[nH]c2cc(Br)ccc12)c1c[nH]c2c(Br)c(Br)cc(O)c12 Show InChI InChI=1S/C21H19Br3N4O/c1-28-9-16(13-7-27-21-19(13)18(29)5-14(23)20(21)24)26-8-17(28)12-6-25-15-4-10(22)2-3-11(12)15/h2-7,16-17,25-27,29H,8-9H2,1H3/t16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Alpha-1b adrenergic receptor |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287720

(6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...)Show SMILES CN1C[C@@H](NC[C@H]1c1c[nH]c2cc(Br)ccc12)c1c[nH]c2c(Br)c(Br)cc(O)c12 Show InChI InChI=1S/C21H19Br3N4O/c1-28-9-16(13-7-27-21-19(13)18(29)5-14(23)20(21)24)26-8-17(28)12-6-25-15-4-10(22)2-3-11(12)15/h2-7,16-17,25-27,29H,8-9H2,1H3/t16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287718
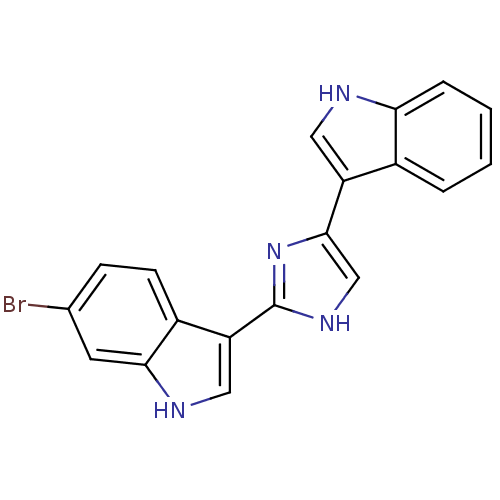
(6-Bromo-3-[4-(1H-indol-3-yl)-1H-imidazol-2-yl]-1H-...)Show SMILES Brc1ccc2c(c[nH]c2c1)-c1nc(c[nH]1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C19H13BrN4/c20-11-5-6-13-15(9-22-17(13)7-11)19-23-10-18(24-19)14-8-21-16-4-2-1-3-12(14)16/h1-10,21-22H,(H,23,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50287720

(6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...)Show SMILES CN1C[C@@H](NC[C@H]1c1c[nH]c2cc(Br)ccc12)c1c[nH]c2c(Br)c(Br)cc(O)c12 Show InChI InChI=1S/C21H19Br3N4O/c1-28-9-16(13-7-27-21-19(13)18(29)5-14(23)20(21)24)26-8-17(28)12-6-25-15-4-10(22)2-3-11(12)15/h2-7,16-17,25-27,29H,8-9H2,1H3/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity was determined against Alpha-1a adrenergic receptor |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50287720

(6,7-Dibromo-3-[(2S,5R)-5-(6-bromo-1H-indol-3-yl)-4...)Show SMILES CN1C[C@@H](NC[C@H]1c1c[nH]c2cc(Br)ccc12)c1c[nH]c2c(Br)c(Br)cc(O)c12 Show InChI InChI=1S/C21H19Br3N4O/c1-28-9-16(13-7-27-21-19(13)18(29)5-14(23)20(21)24)26-8-17(28)12-6-25-15-4-10(22)2-3-11(12)15/h2-7,16-17,25-27,29H,8-9H2,1H3/t16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287719
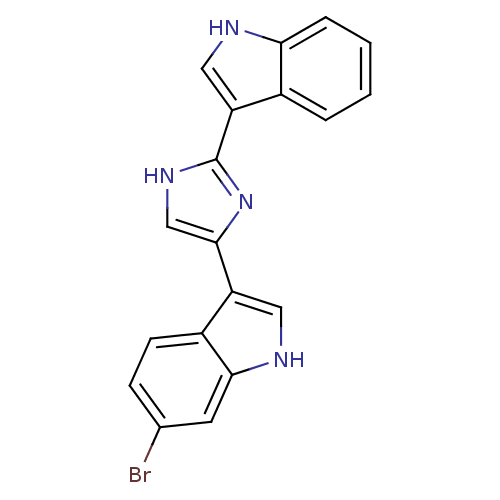
(6-Bromo-3-[2-(1H-indol-3-yl)-1H-imidazol-4-yl]-1H-...)Show SMILES Brc1ccc2c(c[nH]c2c1)-c1c[nH]c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C19H13BrN4/c20-11-5-6-13-14(8-22-17(13)7-11)18-10-23-19(24-18)15-9-21-16-4-2-1-3-12(15)16/h1-10,21-22H,(H,23,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291683
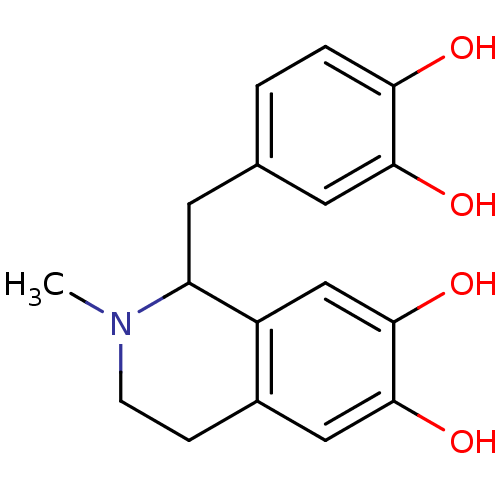
(1-(3,4-Dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C17H19NO4/c1-18-5-4-11-8-16(21)17(22)9-12(11)13(18)6-10-2-3-14(19)15(20)7-10/h2-3,7-9,13,19-22H,4-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681
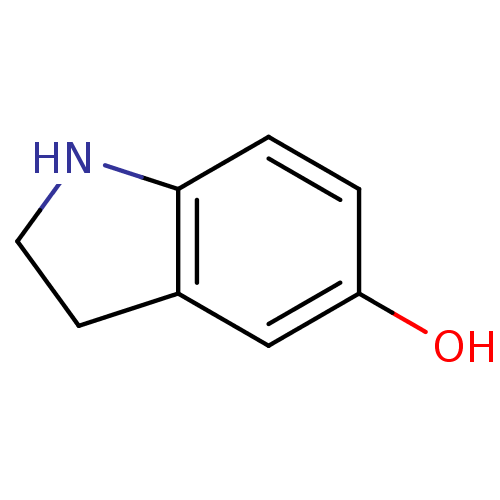
(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287723
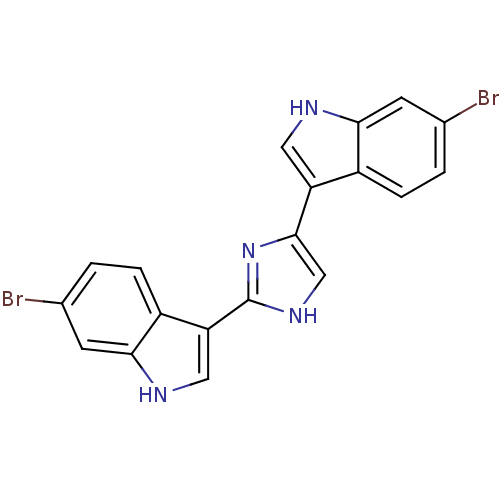
(6-bromo-3-[4-(6-bromo-1H-3-indolyl)-1H-2-imidazoly...)Show SMILES Brc1ccc2c(c[nH]c2c1)-c1c[nH]c(n1)-c1c[nH]c2cc(Br)ccc12 Show InChI InChI=1S/C19H12Br2N4/c20-10-1-3-12-14(7-22-16(12)5-10)18-9-24-19(25-18)15-8-23-17-6-11(21)2-4-13(15)17/h1-9,22-23H,(H,24,25) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50287719

(6-Bromo-3-[2-(1H-indol-3-yl)-1H-imidazol-4-yl]-1H-...)Show SMILES Brc1ccc2c(c[nH]c2c1)-c1c[nH]c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C19H13BrN4/c20-11-5-6-13-14(8-22-17(13)7-11)18-10-23-19(24-18)15-9-21-16-4-2-1-3-12(15)16/h1-10,21-22H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287722
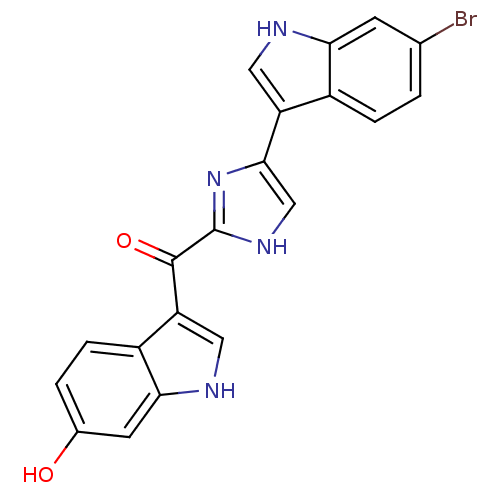
(CHEMBL436760 | [4-(6-Bromo-1H-indol-3-yl)-1H-imida...)Show SMILES Oc1ccc2c(c[nH]c2c1)C(=O)c1nc(c[nH]1)-c1c[nH]c2cc(Br)ccc12 Show InChI InChI=1S/C20H13BrN4O2/c21-10-1-3-12-14(7-22-16(12)5-10)18-9-24-20(25-18)19(27)15-8-23-17-6-11(26)2-4-13(15)17/h1-9,22-23,26H,(H,24,25) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50287718

(6-Bromo-3-[4-(1H-indol-3-yl)-1H-imidazol-2-yl]-1H-...)Show SMILES Brc1ccc2c(c[nH]c2c1)-c1nc(c[nH]1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C19H13BrN4/c20-11-5-6-13-15(9-22-17(13)7-11)19-23-10-18(24-19)14-8-21-16-4-2-1-3-12(14)16/h1-10,21-22H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50287721
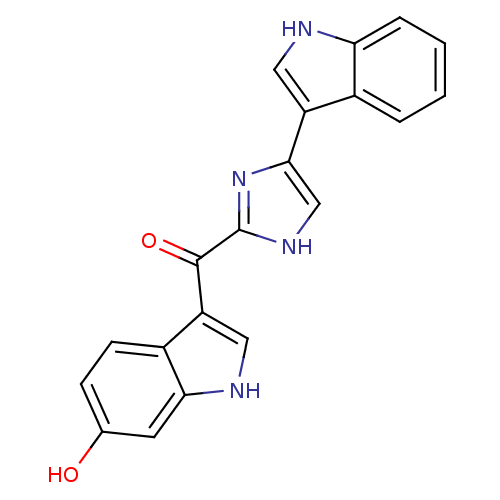
((6-Hydroxy-1H-indol-3-yl)-[5-(1H-indol-3-yl)-1H-im...)Show SMILES Oc1ccc2c(c[nH]c2c1)C(=O)c1nc(c[nH]1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H14N4O2/c25-11-5-6-13-15(9-22-17(13)7-11)19(26)20-23-10-18(24-20)14-8-21-16-4-2-1-3-12(14)16/h1-10,21-22,25H,(H,23,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster Alpha-1b adrenergic receptor by displacing [125I]-HEAT (2-... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614
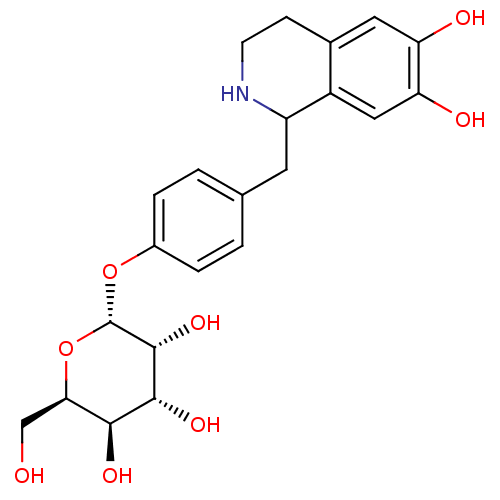
(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291683

(1-(3,4-Dihydroxy-benzyl)-2-methyl-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C17H19NO4/c1-18-5-4-11-8-16(21)17(22)9-12(11)13(18)6-10-2-3-14(19)15(20)7-10/h2-3,7-9,13,19-22H,4-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50287723

(6-bromo-3-[4-(6-bromo-1H-3-indolyl)-1H-2-imidazoly...)Show SMILES Brc1ccc2c(c[nH]c2c1)-c1c[nH]c(n1)-c1c[nH]c2cc(Br)ccc12 Show InChI InChI=1S/C19H12Br2N4/c20-10-1-3-12-14(7-22-16(12)5-10)18-9-24-19(25-18)15-8-23-17-6-11(21)2-4-13(15)17/h1-9,22-23H,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027331
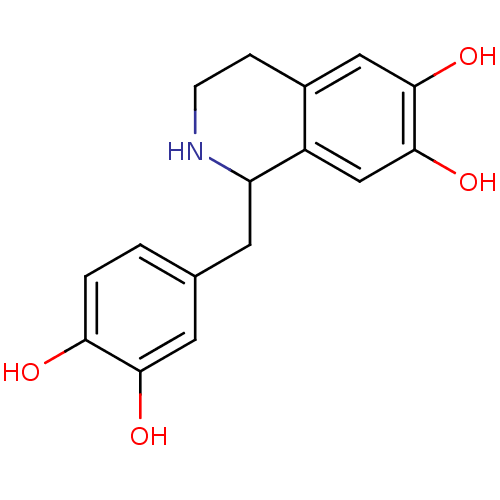
(1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquino...)Show InChI InChI=1S/C16H17NO4/c18-13-2-1-9(6-14(13)19)5-12-11-8-16(21)15(20)7-10(11)3-4-17-12/h1-2,6-8,12,17-21H,3-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50242856
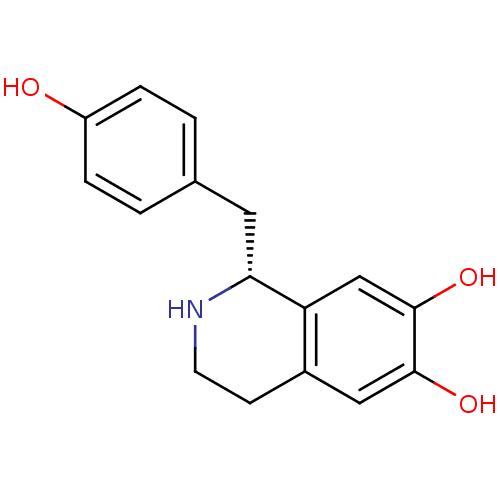
((1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquin...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)7-14-13-9-16(20)15(19)8-11(13)5-6-17-14/h1-4,8-9,14,17-20H,5-7H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027331

(1-(3,4-dihydroxybenzyl)-1,2,3,4-tetrahydroisoquino...)Show InChI InChI=1S/C16H17NO4/c18-13-2-1-9(6-14(13)19)5-12-11-8-16(21)15(20)7-10(11)3-4-17-12/h1-2,6-8,12,17-21H,3-5H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50242856

((1R)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquin...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)7-14-13-9-16(20)15(19)8-11(13)5-6-17-14/h1-4,8-9,14,17-20H,5-7H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50287721

((6-Hydroxy-1H-indol-3-yl)-[5-(1H-indol-3-yl)-1H-im...)Show SMILES Oc1ccc2c(c[nH]c2c1)C(=O)c1nc(c[nH]1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C20H14N4O2/c25-11-5-6-13-15(9-22-17(13)7-11)19(26)20-23-10-18(24-20)14-8-21-16-4-2-1-3-12(14)16/h1-10,21-22,25H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards Rat-1 cells stably expressing hamster alpha1b adrenergic receptor by displacing [125I]-HEAT (2-b... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291684

(1-Methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol ...)Show InChI InChI=1S/C10H13NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-6,11-13H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50291684

(1-Methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol ...)Show InChI InChI=1S/C10H13NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-6,11-13H,2-3H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50291681

(2,3-Dihydro-1H-indol-5-ol | CHEMBL19331)Show InChI InChI=1S/C8H9NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-2,5,9-10H,3-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50450614

(CHEMBL2303762)Show SMILES OC[C@H]1O[C@H](Oc2ccc(CC3NCCc4cc(O)c(O)cc34)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H27NO8/c24-10-18-19(27)20(28)21(29)22(31-18)30-13-3-1-11(2-4-13)7-15-14-9-17(26)16(25)8-12(14)5-6-23-15/h1-4,8-9,15,18-29H,5-7,10H2/t15?,18-,19+,20-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50287722

(CHEMBL436760 | [4-(6-Bromo-1H-indol-3-yl)-1H-imida...)Show SMILES Oc1ccc2c(c[nH]c2c1)C(=O)c1nc(c[nH]1)-c1c[nH]c2cc(Br)ccc12 Show InChI InChI=1S/C20H13BrN4O2/c21-10-1-3-12-14(7-22-16(12)5-10)18-9-24-20(25-18)19(27)15-8-23-17-6-11(26)2-4-13(15)17/h1-9,22-23,26H,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards CHO cells expressing human Alpha-1a adrenergic receptor by displacing [125I]-HEAT (2-beta-(4-hyd... |
Bioorg Med Chem Lett 6: 2103-2106 (1996)
Article DOI: 10.1016/0960-894X(96)00376-9
BindingDB Entry DOI: 10.7270/Q2SJ1KMR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]spiperone binding to human D2 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Potency was measured by the displacement of [3H]-spiperone binding to human D4 dopaminergic receptor |
Bioorg Med Chem Lett 7: 1207-1212 (1997)
Article DOI: 10.1016/S0960-894X(97)00194-7
BindingDB Entry DOI: 10.7270/Q2CJ8F0Z |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437357
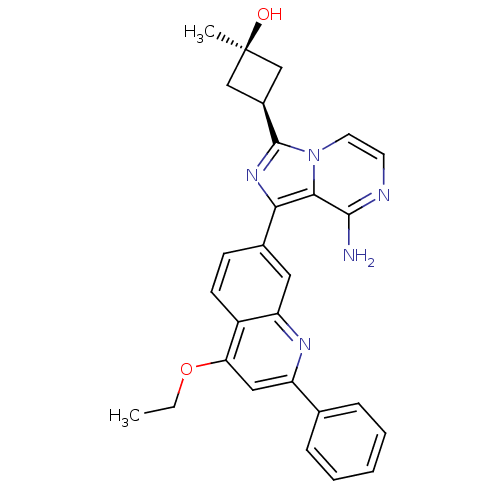
(CHEMBL3037909)Show SMILES CCOc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(12.1,-.5,;13.44,-1.27,;13.44,-2.81,;14.77,-3.58,;16.1,-2.81,;17.44,-3.57,;17.45,-5.13,;16.11,-5.9,;16.11,-7.43,;14.79,-8.21,;13.44,-7.44,;13.44,-5.9,;14.77,-5.13,;14.8,-9.75,;16.05,-10.65,;15.58,-12.12,;16.49,-13.36,;18.01,-13.59,;17.77,-15.11,;19.26,-15.51,;18.17,-16.6,;16.26,-14.88,;14.04,-12.13,;13.02,-13.27,;11.51,-12.95,;11.04,-11.49,;12.07,-10.35,;11.59,-8.88,;13.56,-10.67,;18.77,-2.8,;20.11,-3.57,;21.44,-2.8,;21.43,-1.25,;20.09,-.49,;18.76,-1.27,)| Show InChI InChI=1S/C28H27N5O2/c1-3-35-23-14-21(17-7-5-4-6-8-17)31-22-13-18(9-10-20(22)23)24-25-26(29)30-11-12-33(25)27(32-24)19-15-28(2,34)16-19/h4-14,19,34H,3,15-16H2,1-2H3,(H2,29,30)/t19-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336325
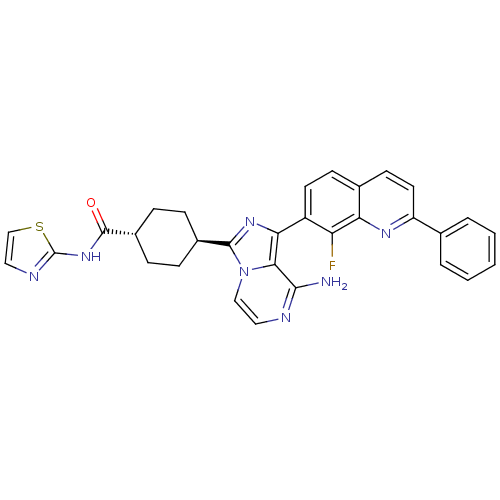
(CHEMBL1667943 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nccs1 |r,wU:27.31,wD:30.38,(5.48,2.11,;5.49,.57,;4.16,-.2,;4.16,-1.75,;5.49,-2.52,;6.83,-1.74,;8.3,-2.21,;9.2,-.95,;8.29,.29,;9.06,1.62,;8.29,2.94,;9.05,4.28,;10.6,4.28,;11.36,5.6,;12.89,5.61,;13.67,4.27,;12.9,2.95,;11.37,2.95,;10.6,1.61,;11.37,.28,;15.2,4.27,;15.97,2.94,;17.51,2.94,;18.28,4.27,;17.5,5.61,;15.96,5.6,;6.82,-.2,;8.79,-3.67,;10.29,-3.97,;10.77,-5.44,;9.74,-6.59,;8.24,-6.27,;7.76,-4.81,;10.22,-8.05,;9.44,-9.37,;11.76,-8.07,;12.52,-9.41,;12.05,-10.87,;13.3,-11.77,;14.54,-10.87,;14.07,-9.4,)| Show InChI InChI=1S/C31H26FN7OS/c32-24-22(12-10-19-11-13-23(36-25(19)24)18-4-2-1-3-5-18)26-27-28(33)34-14-16-39(27)29(37-26)20-6-8-21(9-7-20)30(40)38-31-35-15-17-41-31/h1-5,10-17,20-21H,6-9H2,(H2,33,34)(H,35,38,40)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336329
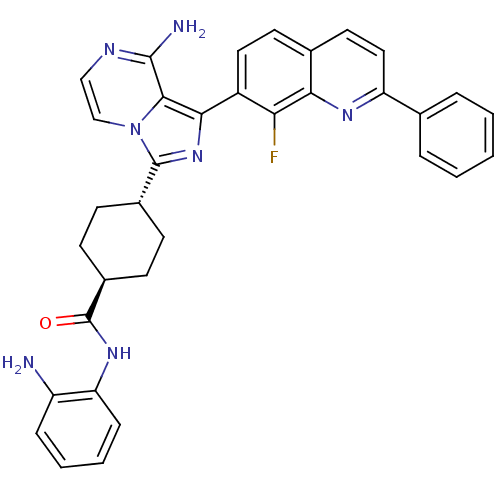
(CHEMBL1667947 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1ccccc1NC(=O)[C@H]1CC[C@@H](CC1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:13.17,wD:10.10,(28.81,-26.74,;28.05,-25.39,;28.84,-24.07,;28.08,-22.73,;26.54,-22.71,;25.76,-24.04,;26.52,-25.37,;25.73,-26.69,;24.19,-26.68,;23.41,-28,;23.71,-25.21,;24.74,-24.06,;24.26,-22.6,;22.75,-22.3,;21.73,-23.44,;22.21,-24.89,;22.27,-20.83,;23.17,-19.58,;22.26,-18.34,;23.03,-17.01,;22.26,-15.69,;23.02,-14.35,;24.57,-14.35,;25.33,-13.03,;26.86,-13.02,;27.63,-14.35,;26.87,-15.68,;25.33,-15.68,;24.57,-17.02,;25.34,-18.35,;29.17,-14.35,;29.94,-15.69,;31.48,-15.69,;32.25,-14.36,;31.47,-13.02,;29.93,-13.02,;20.79,-18.82,;19.46,-18.06,;19.45,-16.52,;18.13,-18.83,;18.13,-20.37,;19.46,-21.15,;20.8,-20.37,)| Show InChI InChI=1S/C34H30FN7O/c35-28-24(16-14-21-15-17-26(39-29(21)28)20-6-2-1-3-7-20)30-31-32(37)38-18-19-42(31)33(41-30)22-10-12-23(13-11-22)34(43)40-27-9-5-4-8-25(27)36/h1-9,14-19,22-23H,10-13,36H2,(H2,37,38)(H,40,43)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437370
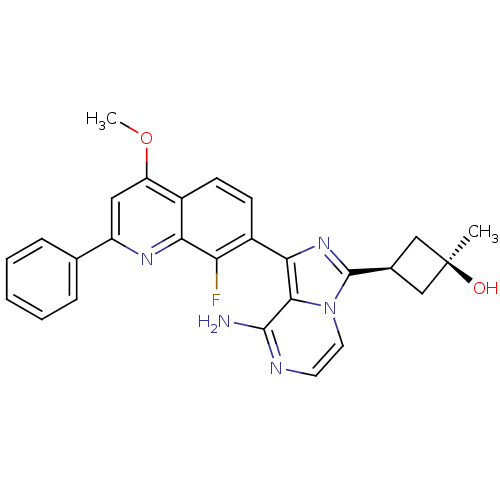
(CHEMBL3037912)Show SMILES COc1cc(nc2c(F)c(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(47.77,-1.38,;47.77,-2.92,;49.1,-3.69,;50.43,-2.92,;51.77,-3.68,;51.78,-5.24,;50.44,-6.01,;50.44,-7.54,;51.78,-8.31,;49.12,-8.32,;47.77,-7.55,;47.77,-6.01,;49.1,-5.24,;49.13,-9.86,;50.38,-10.76,;49.91,-12.23,;50.82,-13.47,;52.34,-13.7,;52.1,-15.22,;53.59,-15.62,;52.5,-16.71,;50.59,-14.99,;48.37,-12.24,;47.35,-13.38,;45.84,-13.06,;45.37,-11.6,;46.4,-10.46,;45.92,-8.99,;47.89,-10.77,;53.1,-2.91,;54.44,-3.68,;55.77,-2.9,;55.76,-1.36,;54.41,-.6,;53.09,-1.37,)| Show InChI InChI=1S/C27H24FN5O2/c1-27(34)13-16(14-27)26-32-23(24-25(29)30-10-11-33(24)26)18-9-8-17-20(35-2)12-19(31-22(17)21(18)28)15-6-4-3-5-7-15/h3-12,16,34H,13-14H2,1-2H3,(H2,29,30)/t16-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437357

(CHEMBL3037909)Show SMILES CCOc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:18.21,16.17,wD:18.20,(12.1,-.5,;13.44,-1.27,;13.44,-2.81,;14.77,-3.58,;16.1,-2.81,;17.44,-3.57,;17.45,-5.13,;16.11,-5.9,;16.11,-7.43,;14.79,-8.21,;13.44,-7.44,;13.44,-5.9,;14.77,-5.13,;14.8,-9.75,;16.05,-10.65,;15.58,-12.12,;16.49,-13.36,;18.01,-13.59,;17.77,-15.11,;19.26,-15.51,;18.17,-16.6,;16.26,-14.88,;14.04,-12.13,;13.02,-13.27,;11.51,-12.95,;11.04,-11.49,;12.07,-10.35,;11.59,-8.88,;13.56,-10.67,;18.77,-2.8,;20.11,-3.57,;21.44,-2.8,;21.43,-1.25,;20.09,-.49,;18.76,-1.27,)| Show InChI InChI=1S/C28H27N5O2/c1-3-35-23-14-21(17-7-5-4-6-8-17)31-22-13-18(9-10-20(22)23)24-25-26(29)30-11-12-33(25)27(32-24)19-15-28(2,34)16-19/h4-14,19,34H,3,15-16H2,1-2H3,(H2,29,30)/t19-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336321
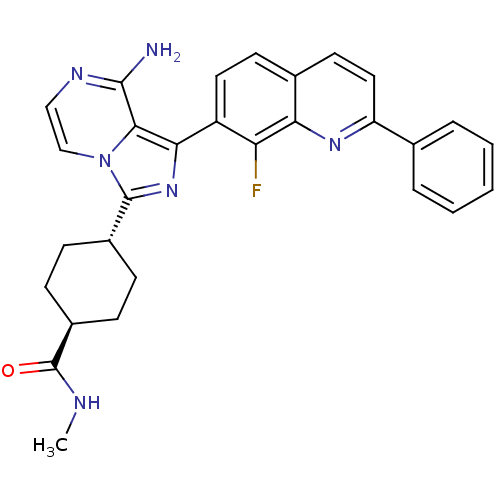
(CHEMBL1667939 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES CNC(=O)[C@H]1CC[C@@H](CC1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:7.10,wD:4.3,(12.87,-39.64,;12.08,-40.96,;10.54,-40.95,;9.76,-42.27,;10.06,-39.48,;11.09,-38.33,;10.61,-36.86,;9.11,-36.56,;8.08,-37.71,;8.56,-39.16,;8.62,-35.1,;9.52,-33.85,;8.61,-32.61,;9.38,-31.28,;8.61,-29.95,;9.37,-28.62,;10.92,-28.62,;11.68,-27.29,;13.21,-27.29,;13.99,-28.62,;13.22,-29.95,;11.69,-29.95,;10.92,-31.28,;11.69,-32.62,;15.52,-28.62,;16.29,-29.96,;17.83,-29.96,;18.6,-28.62,;17.82,-27.29,;16.28,-27.29,;7.14,-33.09,;5.81,-32.33,;5.8,-30.79,;4.48,-33.1,;4.48,-34.64,;5.81,-35.41,;7.15,-34.63,)| Show InChI InChI=1S/C29H27FN6O/c1-32-29(37)20-9-7-19(8-10-20)28-35-25(26-27(31)33-15-16-36(26)28)21-13-11-18-12-14-22(34-24(18)23(21)30)17-5-3-2-4-6-17/h2-6,11-16,19-20H,7-10H2,1H3,(H2,31,33)(H,32,37)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336327
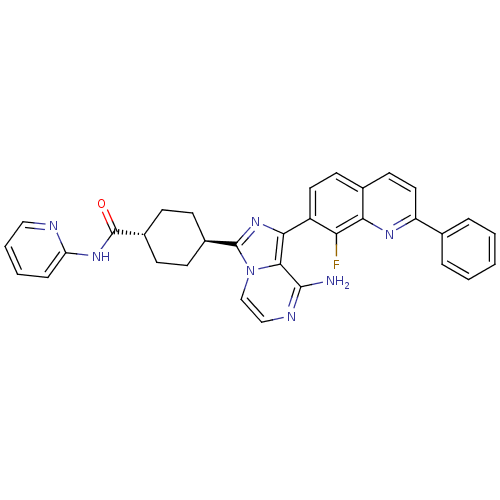
(CHEMBL1667945 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1ccccn1 |r,wU:27.31,wD:30.38,(-8.63,-16.09,;-8.62,-17.63,;-9.95,-18.4,;-9.95,-19.94,;-8.62,-20.71,;-7.28,-19.93,;-5.81,-20.4,;-4.91,-19.15,;-5.82,-17.91,;-5.05,-16.58,;-5.82,-15.25,;-5.06,-13.92,;-3.51,-13.92,;-2.75,-12.59,;-1.22,-12.59,;-.44,-13.92,;-1.21,-15.25,;-2.74,-15.25,;-3.51,-16.58,;-2.74,-17.92,;1.09,-13.92,;1.86,-15.26,;3.4,-15.26,;4.17,-13.92,;3.39,-12.58,;1.85,-12.59,;-7.29,-18.39,;-5.32,-21.86,;-3.82,-22.16,;-3.34,-23.63,;-4.37,-24.78,;-5.87,-24.46,;-6.35,-23.01,;-3.89,-26.25,;-4.67,-27.57,;-2.35,-26.26,;-1.56,-24.94,;-.02,-24.96,;.76,-23.64,;.01,-22.29,;-1.54,-22.28,;-2.32,-23.61,)| Show InChI InChI=1S/C33H28FN7O/c34-27-24(15-13-21-14-16-25(38-28(21)27)20-6-2-1-3-7-20)29-30-31(35)37-18-19-41(30)32(40-29)22-9-11-23(12-10-22)33(42)39-26-8-4-5-17-36-26/h1-8,13-19,22-23H,9-12H2,(H2,35,37)(H,36,39,42)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336324
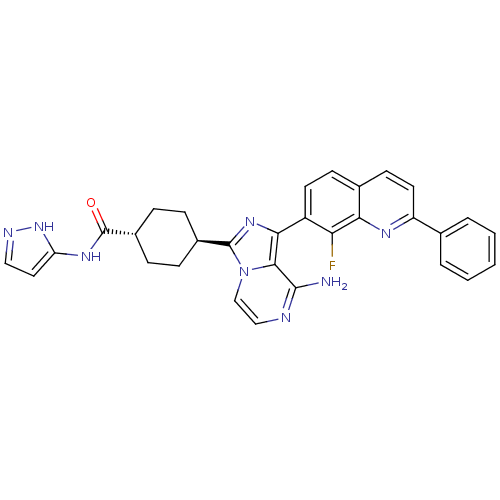
(CHEMBL1667942 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1ccn[nH]1 |r,wU:27.31,wD:30.38,(-8.21,2.51,;-8.21,.97,;-9.54,.19,;-9.54,-1.35,;-8.21,-2.12,;-6.87,-1.34,;-5.4,-1.81,;-4.5,-.56,;-5.41,.69,;-4.64,2.01,;-5.41,3.34,;-4.64,4.67,;-3.1,4.68,;-2.33,6,;-.8,6,;-.03,4.67,;-.8,3.34,;-2.33,3.34,;-3.1,2.01,;-2.33,.67,;1.5,4.67,;2.27,3.33,;3.81,3.33,;4.58,4.67,;3.8,6.01,;2.27,6,;-6.87,.2,;-4.91,-3.27,;-3.4,-3.57,;-2.93,-5.04,;-3.95,-6.19,;-5.46,-5.87,;-5.94,-4.41,;-3.47,-7.65,;-4.26,-8.98,;-1.93,-7.67,;-1.18,-9.01,;.37,-9.01,;.85,-10.47,;-.4,-11.38,;-1.65,-10.47,)| Show InChI InChI=1S/C31H27FN8O/c32-25-22(12-10-19-11-13-23(36-26(19)25)18-4-2-1-3-5-18)27-28-29(33)34-16-17-40(28)30(38-27)20-6-8-21(9-7-20)31(41)37-24-14-15-35-39-24/h1-5,10-17,20-21H,6-9H2,(H2,33,34)(H2,35,37,39,41)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336323

(CHEMBL1667941 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nncs1 |r,wU:27.31,wD:30.38,(36.37,-27.21,;36.37,-28.75,;35.04,-29.52,;35.04,-31.06,;36.38,-31.83,;37.72,-31.05,;39.19,-31.52,;40.09,-30.27,;39.17,-29.02,;39.94,-27.7,;39.17,-26.37,;39.94,-25.04,;41.49,-25.04,;42.25,-23.71,;43.78,-23.71,;44.55,-25.04,;43.78,-26.37,;42.25,-26.37,;41.48,-27.7,;42.25,-29.04,;46.09,-25.04,;46.85,-26.38,;48.39,-26.38,;49.16,-25.04,;48.39,-23.7,;46.85,-23.71,;37.71,-29.51,;39.67,-32.98,;41.18,-33.28,;41.66,-34.75,;40.63,-35.9,;39.12,-35.58,;38.64,-34.12,;41.11,-37.36,;40.32,-38.69,;42.65,-37.38,;43.4,-38.72,;44.94,-38.9,;45.24,-40.41,;43.9,-41.16,;42.77,-40.12,)| Show InChI InChI=1S/C30H25FN8OS/c31-23-21(12-10-18-11-13-22(35-24(18)23)17-4-2-1-3-5-17)25-26-27(32)33-14-15-39(26)28(36-25)19-6-8-20(9-7-19)29(40)37-30-38-34-16-41-30/h1-5,10-16,19-20H,6-9H2,(H2,32,33)(H,37,38,40)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437369
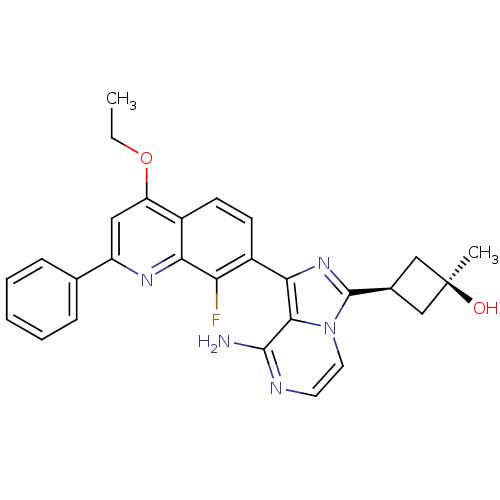
(CHEMBL3037913)Show SMILES CCOc1cc(nc2c(F)c(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:19.22,17.18,wD:19.21,(23.32,-.37,;24.65,-1.14,;24.65,-2.69,;25.99,-3.46,;27.32,-2.69,;28.65,-3.45,;28.66,-5,;27.32,-5.77,;27.32,-7.31,;28.66,-8.07,;26,-8.08,;24.65,-7.32,;24.65,-5.77,;25.98,-5,;26.01,-9.63,;27.26,-10.53,;26.79,-11.99,;27.7,-13.24,;29.23,-13.47,;28.99,-14.99,;30.47,-15.38,;29.39,-16.47,;27.47,-14.76,;25.25,-12,;24.23,-13.15,;22.72,-12.82,;22.25,-11.36,;23.28,-10.22,;22.8,-8.76,;24.77,-10.54,;29.98,-2.68,;31.32,-3.45,;32.65,-2.67,;32.64,-1.13,;31.3,-.36,;29.97,-1.14,)| Show InChI InChI=1S/C28H26FN5O2/c1-3-36-21-13-20(16-7-5-4-6-8-16)32-23-18(21)9-10-19(22(23)29)24-25-26(30)31-11-12-34(25)27(33-24)17-14-28(2,35)15-17/h4-13,17,35H,3,14-15H2,1-2H3,(H2,30,31)/t17-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of full length human IGF-1R overexpressed in mouse NIH 3T3 cells assessed as IGF1-induced protein phosphorylation incubated for 2 hrs prio... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336315
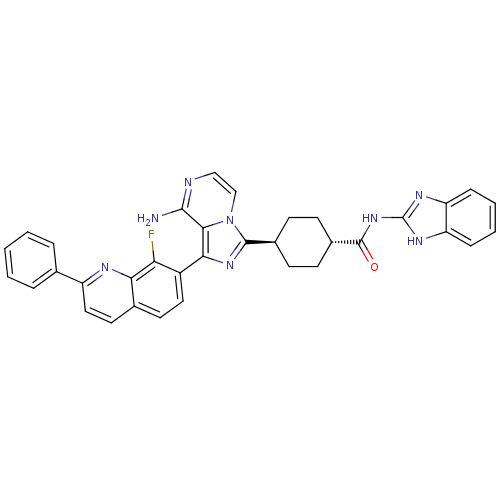
(CHEMBL1667948 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccccc2[nH]1 |r,wU:27.31,wD:30.38,(-9.22,-32.11,;-9.21,-33.66,;-10.54,-34.43,;-10.55,-35.97,;-9.21,-36.74,;-7.87,-35.96,;-6.4,-36.43,;-5.5,-35.18,;-6.41,-33.93,;-5.64,-32.61,;-6.41,-31.28,;-5.64,-29.95,;-4.09,-29.94,;-3.33,-28.62,;-1.8,-28.61,;-1.03,-29.95,;-1.8,-31.28,;-3.33,-31.27,;-4.1,-32.61,;-3.33,-33.95,;.51,-29.95,;1.28,-31.28,;2.82,-31.28,;3.59,-29.95,;2.81,-28.61,;1.27,-28.62,;-7.88,-34.42,;-5.91,-37.9,;-4.4,-38.2,;-3.92,-39.67,;-4.95,-40.82,;-6.46,-40.5,;-6.94,-39.04,;-4.47,-42.28,;-5.26,-43.61,;-2.93,-42.3,;-2.15,-40.97,;-2.75,-39.55,;-1.6,-38.53,;-1.58,-37,;-.25,-36.25,;1.08,-37.03,;1.06,-38.56,;-.27,-39.31,;-.61,-40.82,)| Show InChI InChI=1S/C35H29FN8O/c36-28-24(16-14-21-15-17-25(39-29(21)28)20-6-2-1-3-7-20)30-31-32(37)38-18-19-44(31)33(42-30)22-10-12-23(13-11-22)34(45)43-35-40-26-8-4-5-9-27(26)41-35/h1-9,14-19,22-23H,10-13H2,(H2,37,38)(H2,40,41,43,45)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336322
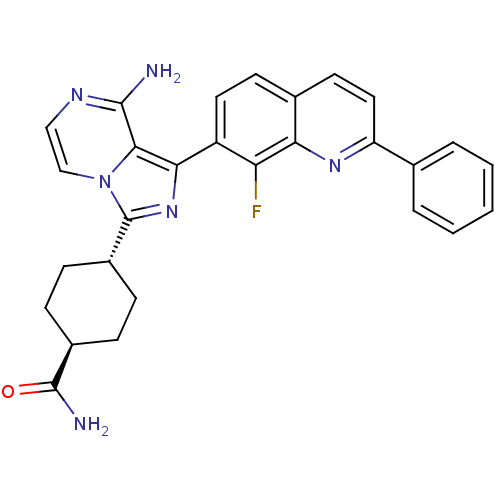
(CHEMBL1667940 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES NC(=O)[C@H]1CC[C@@H](CC1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:6.9,wD:3.2,(26.07,-41.48,;24.53,-41.46,;23.74,-42.79,;24.05,-40,;25.07,-38.85,;24.6,-37.38,;23.09,-37.08,;22.06,-38.22,;22.54,-39.68,;22.6,-35.62,;23.5,-34.37,;22.59,-33.12,;23.36,-31.8,;22.59,-30.47,;23.36,-29.14,;24.9,-29.13,;25.67,-27.81,;27.2,-27.81,;27.97,-29.14,;27.2,-30.47,;25.67,-30.47,;24.9,-31.8,;25.67,-33.14,;29.5,-29.14,;30.27,-30.48,;31.81,-30.48,;32.58,-29.14,;31.8,-27.8,;30.27,-27.81,;21.13,-33.61,;19.79,-32.84,;19.79,-31.3,;18.46,-33.62,;18.46,-35.16,;19.79,-35.93,;21.13,-35.15,)| Show InChI InChI=1S/C28H25FN6O/c29-22-20(12-10-17-11-13-21(33-23(17)22)16-4-2-1-3-5-16)24-25-26(30)32-14-15-35(25)28(34-24)19-8-6-18(7-9-19)27(31)36/h1-5,10-15,18-19H,6-9H2,(H2,30,32)(H2,31,36)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50437358

(CHEMBL3037908)Show SMILES COc1cc(nc2cc(ccc12)-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12)-c1ccccc1 |r,wU:17.20,15.16,wD:17.19,(2.3,-.85,;2.3,-2.39,;3.63,-3.16,;4.96,-2.39,;6.3,-3.15,;6.31,-4.71,;4.97,-5.48,;4.97,-7.01,;3.65,-7.79,;2.3,-7.02,;2.3,-5.48,;3.63,-4.71,;3.66,-9.33,;4.91,-10.23,;4.44,-11.7,;5.35,-12.94,;6.87,-13.17,;6.63,-14.69,;8.12,-15.09,;7.03,-16.18,;5.12,-14.46,;2.9,-11.71,;1.88,-12.85,;.37,-12.53,;-.11,-11.07,;.93,-9.93,;.45,-8.46,;2.42,-10.25,;7.63,-2.38,;8.97,-3.15,;10.3,-2.38,;10.29,-.83,;8.94,-.07,;7.62,-.85,)| Show InChI InChI=1S/C27H25N5O2/c1-27(33)14-18(15-27)26-31-23(24-25(28)29-10-11-32(24)26)17-8-9-19-21(12-17)30-20(13-22(19)34-2)16-6-4-3-5-7-16/h3-13,18,33H,14-15H2,1-2H3,(H2,28,29)/t18-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF-1R catalytic domain (unknown origin) using omnia Y peptide-12 as substrate assessed as inhibition of substrate phosphory... |
ACS Med Chem Lett 4: 627-31 (2013)
Article DOI: 10.1021/ml400160a
BindingDB Entry DOI: 10.7270/Q2S75HR4 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339062
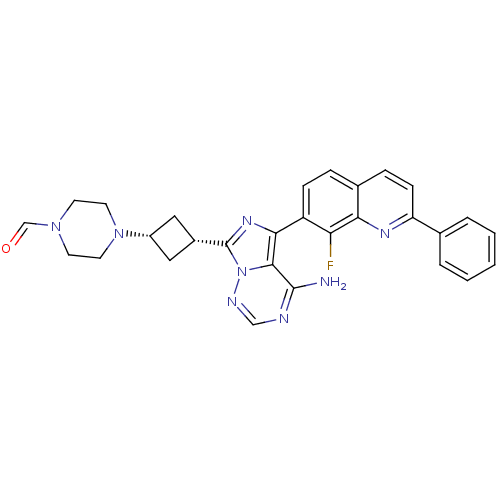
(4-(3-(4-amino-5-(8-fluoro-2-phenylquinolin-7-yl)im...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:27.31,29.36,(5.56,-13.24,;5.57,-14.78,;4.24,-15.55,;4.24,-17.09,;5.57,-17.86,;6.9,-17.08,;8.37,-17.56,;9.28,-16.31,;8.37,-15.06,;9.12,-13.72,;8.34,-12.4,;9.1,-11.06,;10.64,-11.05,;11.4,-9.71,;12.94,-9.7,;13.72,-11.04,;12.95,-12.37,;11.41,-12.38,;10.67,-13.71,;11.45,-15.04,;15.25,-11.03,;16.03,-12.36,;17.57,-12.35,;18.33,-11.01,;17.55,-9.68,;16.01,-9.69,;6.9,-15.54,;8.84,-19.01,;8.14,-20.39,;9.52,-21.09,;10.21,-19.71,;10,-22.55,;8.96,-23.7,;9.43,-25.15,;10.94,-25.48,;11.97,-24.34,;11.5,-22.87,;11.41,-26.95,;10.37,-28.09,)| Show InChI InChI=1S/C29H27FN8O/c30-24-22(8-6-19-7-9-23(34-25(19)24)18-4-2-1-3-5-18)26-27-28(31)32-16-33-38(27)29(35-26)20-14-21(15-20)37-12-10-36(17-39)11-13-37/h1-9,16-17,20-21H,10-15H2,(H2,31,32,33)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data