 Found 2007 hits with Last Name = 'yao' and Initial = 'y'
Found 2007 hits with Last Name = 'yao' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396023
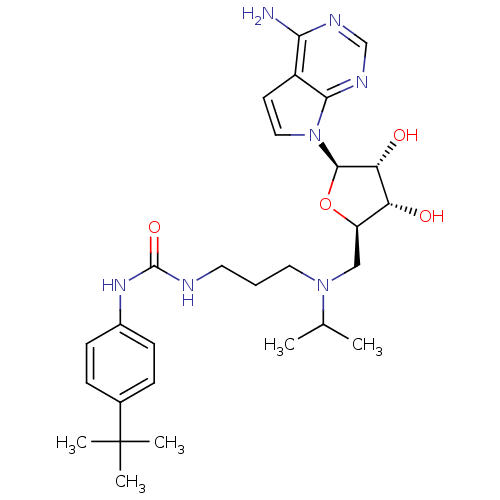
(CHEMBL2169919)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980

(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980

(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396979
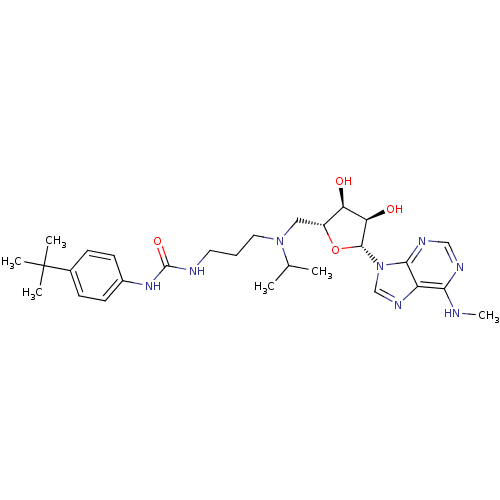
(CHEMBL2171170)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCCNC(=O)Nc2ccc(cc2)C(C)(C)C)C(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H42N8O4/c1-17(2)35(13-7-12-30-27(39)34-19-10-8-18(9-11-19)28(3,4)5)14-20-22(37)23(38)26(40-20)36-16-33-21-24(29-6)31-15-32-25(21)36/h8-11,15-17,20,22-23,26,37-38H,7,12-14H2,1-6H3,(H,29,31,32)(H2,30,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50443018
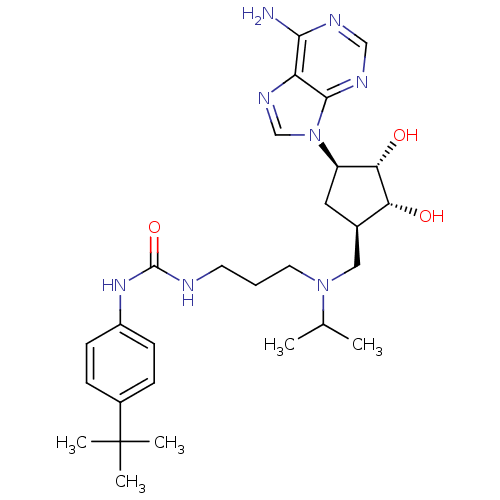
(CHEMBL3087503)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H42N8O3/c1-17(2)35(12-6-11-30-27(39)34-20-9-7-19(8-10-20)28(3,4)5)14-18-13-21(24(38)23(18)37)36-16-33-22-25(29)31-15-32-26(22)36/h7-10,15-18,21,23-24,37-38H,6,11-14H2,1-5H3,(H2,29,31,32)(H2,30,34,39)/t18-,21-,23-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50443019
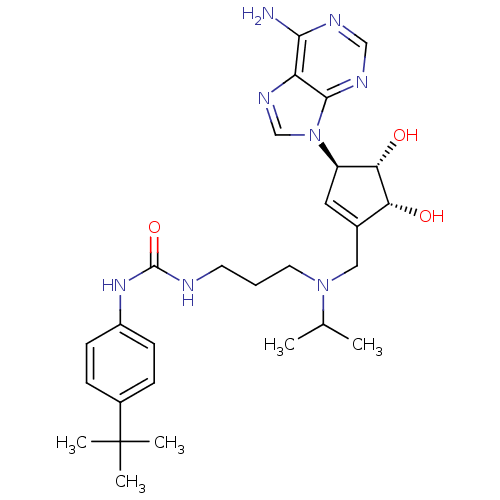
(CHEMBL3087502)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1=C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r,t:23| Show InChI InChI=1S/C28H40N8O3/c1-17(2)35(12-6-11-30-27(39)34-20-9-7-19(8-10-20)28(3,4)5)14-18-13-21(24(38)23(18)37)36-16-33-22-25(29)31-15-32-26(22)36/h7-10,13,15-17,21,23-24,37-38H,6,11-12,14H2,1-5H3,(H2,29,31,32)(H2,30,34,39)/t21-,23-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM3033

(3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0...)Show SMILES Cn1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4n(CCC#N)c3c12 Show InChI InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM25117

(AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(\C=C\c3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50426474
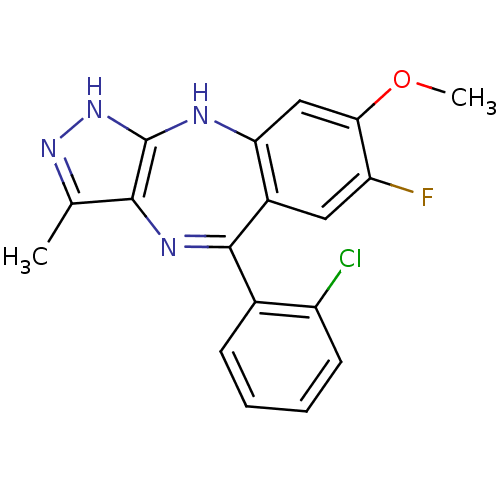
(CHEMBL1980391)Show SMILES COc1cc2Nc3[nH]nc(C)c3N=C(c3ccccc3Cl)c2cc1F |t:13| Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-18(24-23-9)21-14-8-15(25-2)13(20)7-11(14)17(22-16)10-5-3-4-6-12(10)19/h3-8H,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396978
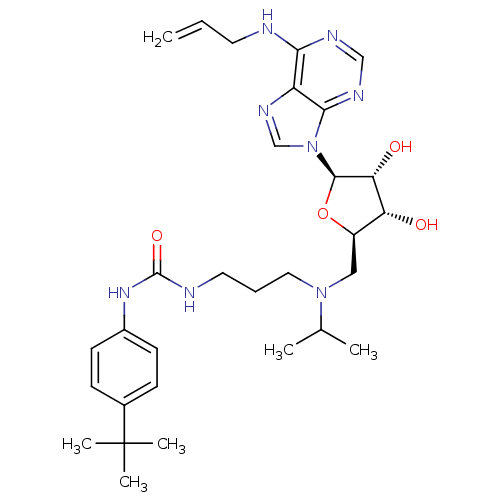
(CHEMBL2171171)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC=C)ncnc12 |r| Show InChI InChI=1S/C30H44N8O4/c1-7-13-31-26-23-27(34-17-33-26)38(18-35-23)28-25(40)24(39)22(42-28)16-37(19(2)3)15-8-14-32-29(41)36-21-11-9-20(10-12-21)30(4,5)6/h7,9-12,17-19,22,24-25,28,39-40H,1,8,13-16H2,2-6H3,(H,31,33,34)(H2,32,36,41)/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396977
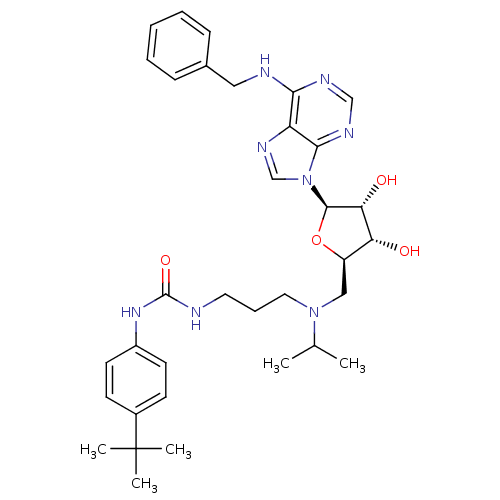
(CHEMBL2171172)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3)ncnc12 |r| Show InChI InChI=1S/C34H46N8O4/c1-22(2)41(17-9-16-35-33(45)40-25-14-12-24(13-15-25)34(3,4)5)19-26-28(43)29(44)32(46-26)42-21-39-27-30(37-20-38-31(27)42)36-18-23-10-7-6-8-11-23/h6-8,10-15,20-22,26,28-29,32,43-44H,9,16-19H2,1-5H3,(H2,35,40,45)(H,36,37,38)/t26-,28-,29-,32-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50437839
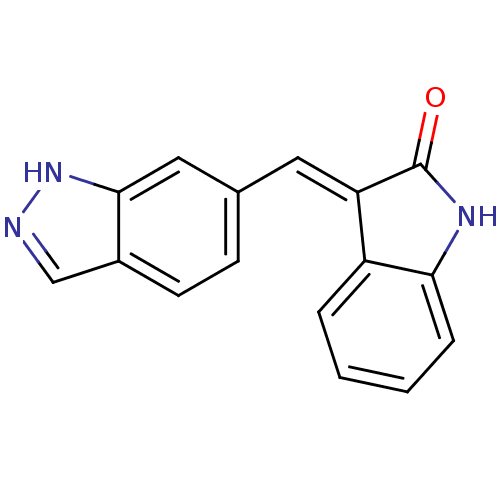
(CHEMBL2407911)Show InChI InChI=1S/C16H11N3O/c20-16-13(12-3-1-2-4-14(12)18-16)7-10-5-6-11-9-17-19-15(11)8-10/h1-9H,(H,17,19)(H,18,20)/b13-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal GST-tagged human PLK4 (1 to 391 amino acids) expressed in Escherichia coli in the presence of ATP |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50437870

(CHEMBL2407901)Show SMILES COCCOc1ccc2Nc3ncnc4[nH]cc(CN(C)CCCN(C)C(=O)COc1c2)c34 Show InChI InChI=1S/C23H30N6O4/c1-28-7-4-8-29(2)20(30)14-33-19-11-17(5-6-18(19)32-10-9-31-3)27-23-21-16(13-28)12-24-22(21)25-15-26-23/h5-6,11-12,15H,4,7-10,13-14H2,1-3H3,(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142238
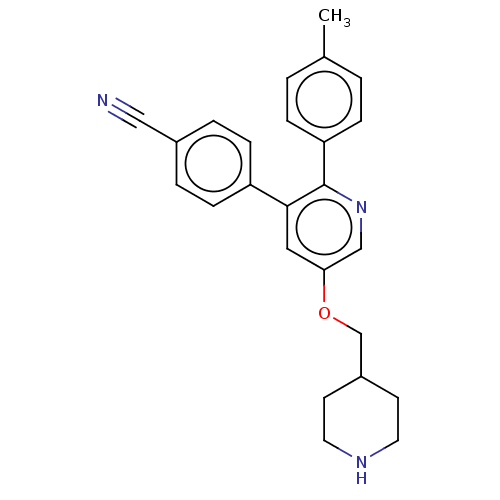
(CHEMBL3759201)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N3O/c1-18-2-6-22(7-3-18)25-24(21-8-4-19(15-26)5-9-21)14-23(16-28-25)29-17-20-10-12-27-13-11-20/h2-9,14,16,20,27H,10-13,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of AXL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142187

(CHEMBL3758634)Show SMILES FC(F)(F)Oc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)33-21-7-5-20(6-8-21)24-23(19-3-1-17(14-29)2-4-19)13-22(15-31-24)32-16-18-9-11-30-12-10-18/h1-8,13,15,18,30H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142237
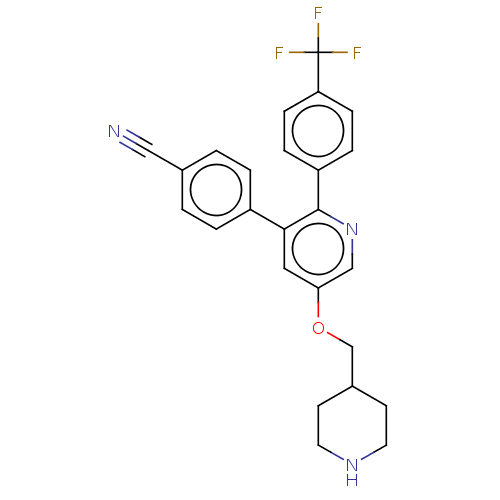
(CHEMBL3759797)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C25H22F3N3O/c26-25(27,28)21-7-5-20(6-8-21)24-23(19-3-1-17(14-29)2-4-19)13-22(15-31-24)32-16-18-9-11-30-12-10-18/h1-8,13,15,18,30H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396988
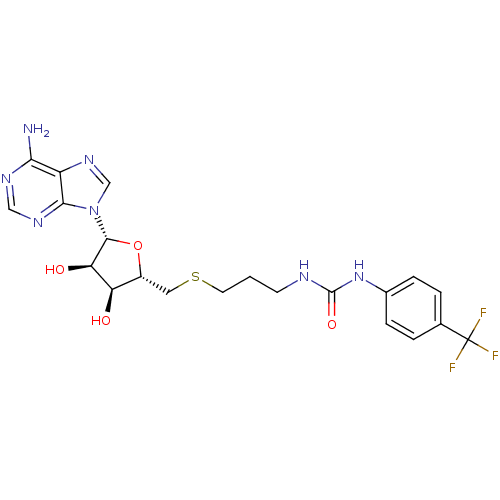
(CHEMBL2170997)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(cc2)C(F)(F)F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24F3N7O4S/c22-21(23,24)11-2-4-12(5-3-11)30-20(34)26-6-1-7-36-8-13-15(32)16(33)19(35-13)31-10-29-14-17(25)27-9-28-18(14)31/h2-5,9-10,13,15-16,19,32-33H,1,6-8H2,(H2,25,27,28)(H2,26,30,34)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396992
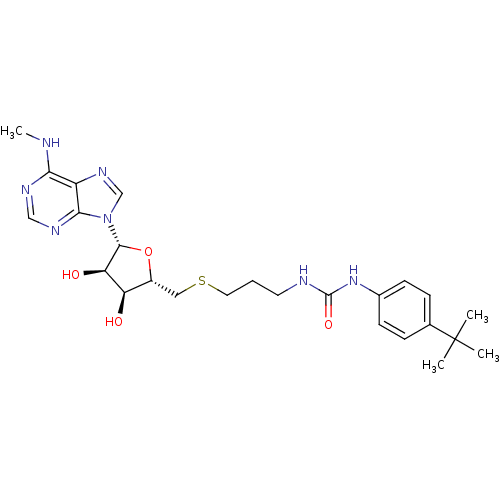
(CHEMBL2170993)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(cc2)C(C)(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C25H35N7O4S/c1-25(2,3)15-6-8-16(9-7-15)31-24(35)27-10-5-11-37-12-17-19(33)20(34)23(36-17)32-14-30-18-21(26-4)28-13-29-22(18)32/h6-9,13-14,17,19-20,23,33-34H,5,10-12H2,1-4H3,(H,26,28,29)(H2,27,31,35)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396983
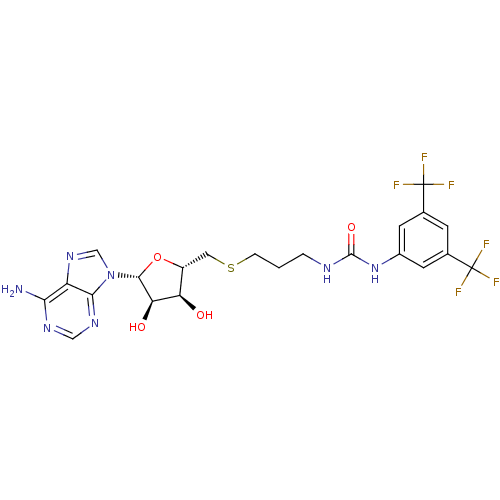
(CHEMBL2171002)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H23F6N7O4S/c23-21(24,25)10-4-11(22(26,27)28)6-12(5-10)34-20(38)30-2-1-3-40-7-13-15(36)16(37)19(39-13)35-9-33-14-17(29)31-8-32-18(14)35/h4-6,8-9,13,15-16,19,36-37H,1-3,7H2,(H2,29,31,32)(H2,30,34,38)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396991
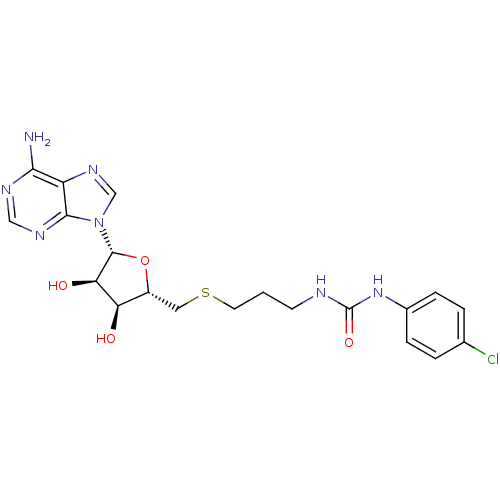
(CHEMBL2170994)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(Cl)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24ClN7O4S/c21-11-2-4-12(5-3-11)27-20(31)23-6-1-7-33-8-13-15(29)16(30)19(32-13)28-10-26-14-17(22)24-9-25-18(14)28/h2-5,9-10,13,15-16,19,29-30H,1,6-8H2,(H2,22,24,25)(H2,23,27,31)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142242
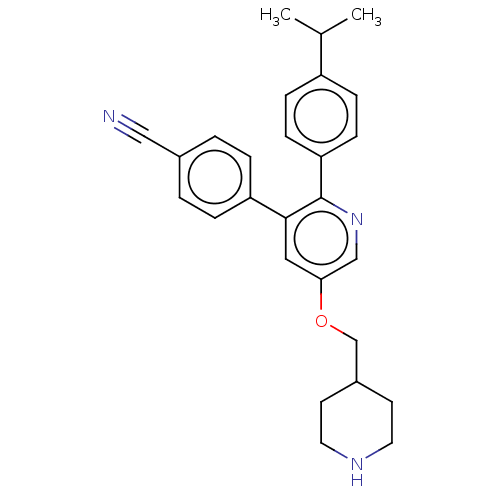
(CHEMBL3759102)Show SMILES CC(C)c1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C27H29N3O/c1-19(2)22-7-9-24(10-8-22)27-26(23-5-3-20(16-28)4-6-23)15-25(17-30-27)31-18-21-11-13-29-14-12-21/h3-10,15,17,19,21,29H,11-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50437869
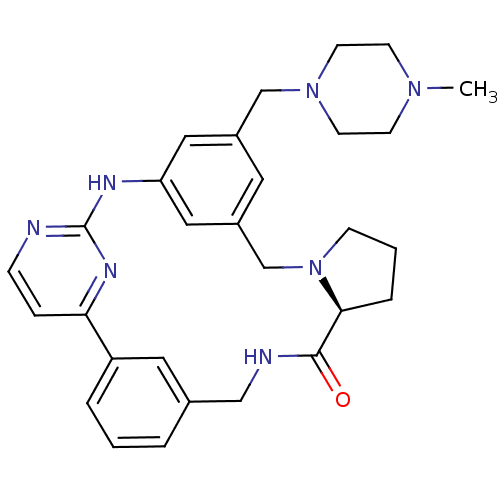
(CHEMBL2407902)Show SMILES CN1CCN(Cc2cc3CN4CCC[C@H]4C(=O)NCc4cccc(c4)-c4ccnc(Nc(c2)c3)n4)CC1 |r| Show InChI InChI=1S/C29H35N7O/c1-34-10-12-35(13-11-34)19-22-14-23-17-25(16-22)32-29-30-8-7-26(33-29)24-5-2-4-21(15-24)18-31-28(37)27-6-3-9-36(27)20-23/h2,4-5,7-8,14-17,27H,3,6,9-13,18-20H2,1H3,(H,31,37)(H,30,32,33)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PLK4 |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50434276
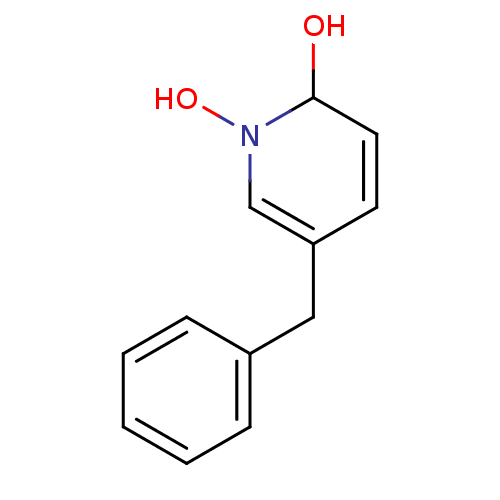
(CHEMBL2386125)Show InChI InChI=1S/C12H13NO2/c14-12-7-6-11(9-13(12)15)8-10-4-2-1-3-5-10/h1-7,9,12,14-15H,8H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human IDH1 R132C mutant expressed in Escherichia coli BL21-CodonPlus assessed as reduction in NADPH consumption |
J Med Chem 57: 8307-18 (2014)
Article DOI: 10.1021/jm500660f
BindingDB Entry DOI: 10.7270/Q23J3FJR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of cMET |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50181153
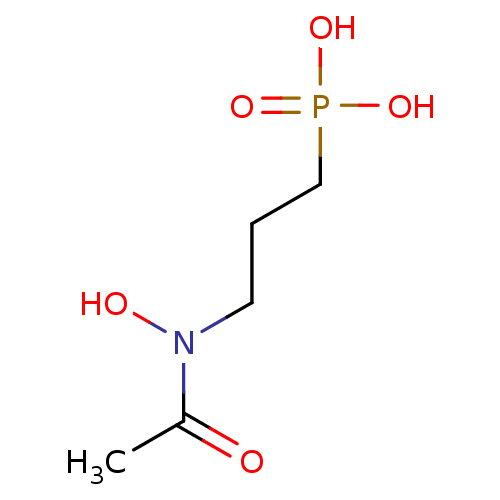
(3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...)Show InChI InChI=1S/C5H12NO5P/c1-5(7)6(8)3-2-4-12(9,10)11/h8H,2-4H2,1H3,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... |
J Med Chem 54: 4721-34 (2011)
Article DOI: 10.1021/jm200363d
BindingDB Entry DOI: 10.7270/Q2NK3FCT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396985
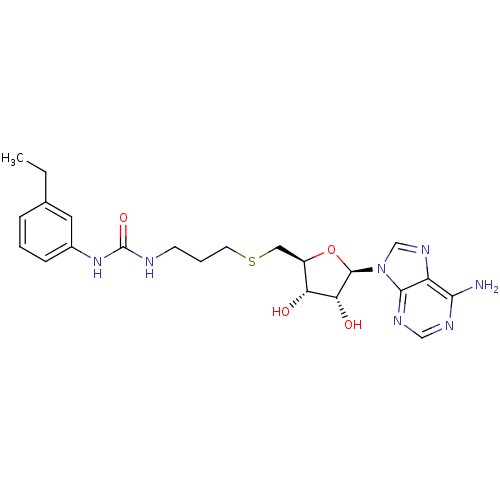
(CHEMBL2171000)Show SMILES CCc1cccc(NC(=O)NCCCSC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)c1 |r| Show InChI InChI=1S/C22H29N7O4S/c1-2-13-5-3-6-14(9-13)28-22(32)24-7-4-8-34-10-15-17(30)18(31)21(33-15)29-12-27-16-19(23)25-11-26-20(16)29/h3,5-6,9,11-12,15,17-18,21,30-31H,2,4,7-8,10H2,1H3,(H2,23,25,26)(H2,24,28,32)/t15-,17-,18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396987
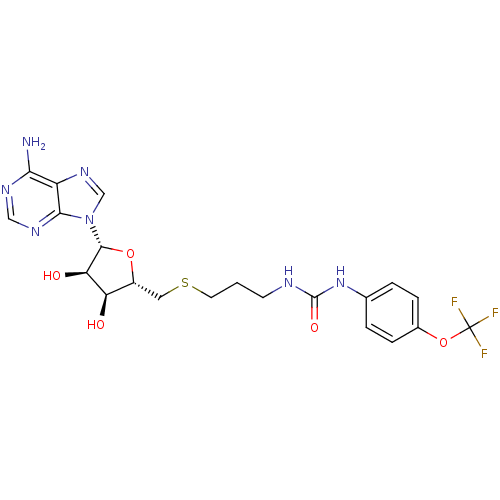
(CHEMBL2170998)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(OC(F)(F)F)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24F3N7O5S/c22-21(23,24)36-12-4-2-11(3-5-12)30-20(34)26-6-1-7-37-8-13-15(32)16(33)19(35-13)31-10-29-14-17(25)27-9-28-18(14)31/h2-5,9-10,13,15-16,19,32-33H,1,6-8H2,(H2,25,27,28)(H2,26,30,34)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50029465
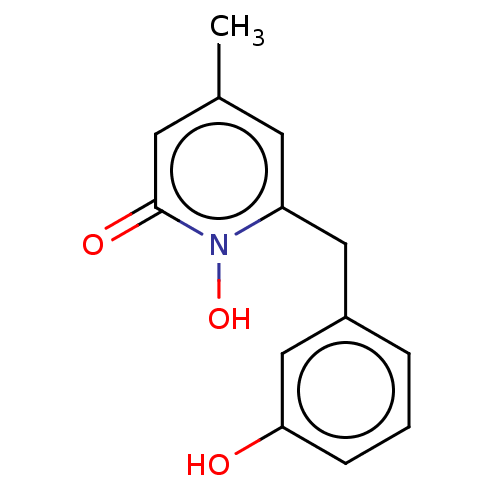
(CHEMBL3343445)Show InChI InChI=1S/C13H13NO3/c1-9-5-11(14(17)13(16)6-9)7-10-3-2-4-12(15)8-10/h2-6,8,15,17H,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human IDH1 R132H mutant expressed in Escherichia coli BL21-CodonPlus assessed as reduction in NADPH consumption |
J Med Chem 57: 8307-18 (2014)
Article DOI: 10.1021/jm500660f
BindingDB Entry DOI: 10.7270/Q23J3FJR |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
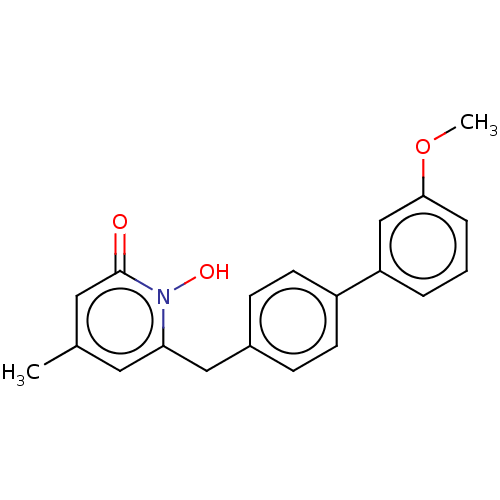
(Homo sapiens (Human)) | BDBM50029491
(CHEMBL3343464)Show InChI InChI=1S/C20H19NO3/c1-14-10-18(21(23)20(22)11-14)12-15-6-8-16(9-7-15)17-4-3-5-19(13-17)24-2/h3-11,13,23H,12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human IDH1 R132H mutant expressed in Escherichia coli BL21-CodonPlus assessed as reduction in NADPH consumption |
J Med Chem 57: 8307-18 (2014)
Article DOI: 10.1021/jm500660f
BindingDB Entry DOI: 10.7270/Q23J3FJR |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Mycobacterium tuberculosis) | BDBM50153713
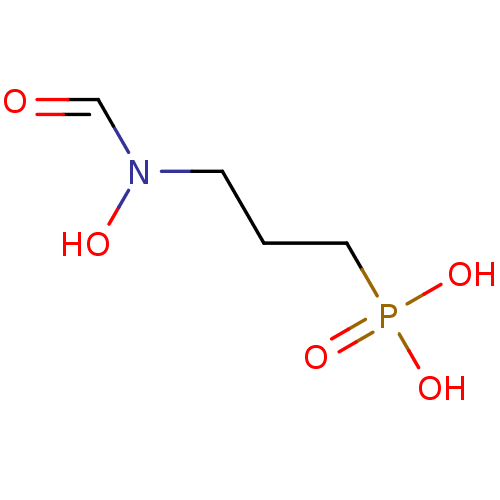
(3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...)Show InChI InChI=1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... |
J Med Chem 54: 4721-34 (2011)
Article DOI: 10.1021/jm200363d
BindingDB Entry DOI: 10.7270/Q2NK3FCT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
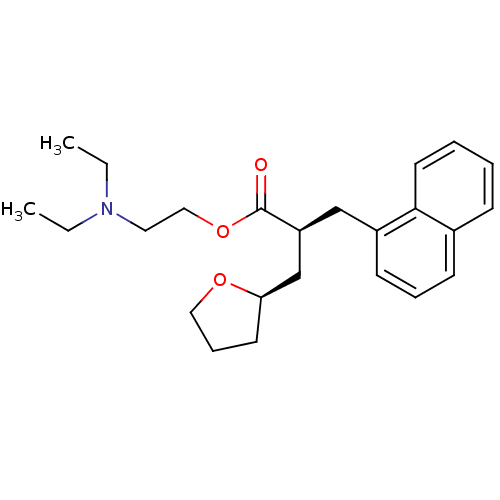
(Homo sapiens (Human)) | BDBM50382173
(CHEMBL2022157)Show SMILES CCN(CC)CCOC(=O)[C@H](C[C@H]1CCCO1)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C24H33NO3/c1-3-25(4-2)14-16-28-24(26)21(18-22-12-8-15-27-22)17-20-11-7-10-19-9-5-6-13-23(19)20/h5-7,9-11,13,21-22H,3-4,8,12,14-18H2,1-2H3/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor expressed in HEK293 cells by liquid scintillation spectrophotometry |
Bioorg Med Chem Lett 22: 3441-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.093
BindingDB Entry DOI: 10.7270/Q2W37XB1 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50139118
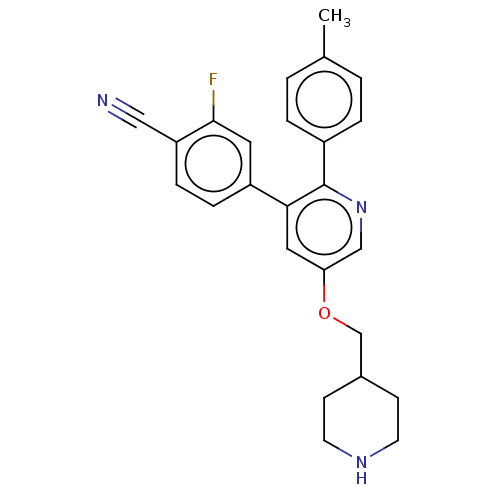
(CHEMBL3759351)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(C#N)c(F)c1 Show InChI InChI=1S/C25H24FN3O/c1-17-2-4-19(5-3-17)25-23(20-6-7-21(14-27)24(26)12-20)13-22(15-29-25)30-16-18-8-10-28-11-9-18/h2-7,12-13,15,18,28H,8-11,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50029463

(CHEMBL3343444)Show InChI InChI=1S/C14H15NO3/c1-10-6-12(15(17)14(16)7-10)8-11-4-3-5-13(9-11)18-2/h3-7,9,17H,8H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human IDH1 R132H mutant expressed in Escherichia coli BL21-CodonPlus assessed as reduction in NADPH consumption |
J Med Chem 57: 8307-18 (2014)
Article DOI: 10.1021/jm500660f
BindingDB Entry DOI: 10.7270/Q23J3FJR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50382173

(CHEMBL2022157)Show SMILES CCN(CC)CCOC(=O)[C@H](C[C@H]1CCCO1)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C24H33NO3/c1-3-25(4-2)14-16-28-24(26)21(18-22-12-8-15-27-22)17-20-11-7-10-19-9-5-6-13-23(19)20/h5-7,9-11,13,21-22H,3-4,8,12,14-18H2,1-2H3/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor expressed in HEK293 cells by liquid scintillation spectrophotometry |
Bioorg Med Chem Lett 22: 3441-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.093
BindingDB Entry DOI: 10.7270/Q2W37XB1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50382170
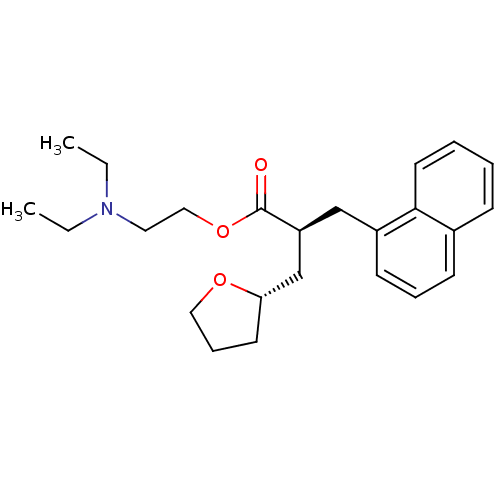
(CHEMBL2022154)Show SMILES CCN(CC)CCOC(=O)[C@H](C[C@@H]1CCCO1)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C24H33NO3/c1-3-25(4-2)14-16-28-24(26)21(18-22-12-8-15-27-22)17-20-11-7-10-19-9-5-6-13-23(19)20/h5-7,9-11,13,21-22H,3-4,8,12,14-18H2,1-2H3/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor expressed in HEK293 cells by liquid scintillation spectrophotometry |
Bioorg Med Chem Lett 22: 3441-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.093
BindingDB Entry DOI: 10.7270/Q2W37XB1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50382170

(CHEMBL2022154)Show SMILES CCN(CC)CCOC(=O)[C@H](C[C@@H]1CCCO1)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C24H33NO3/c1-3-25(4-2)14-16-28-24(26)21(18-22-12-8-15-27-22)17-20-11-7-10-19-9-5-6-13-23(19)20/h5-7,9-11,13,21-22H,3-4,8,12,14-18H2,1-2H3/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor expressed in HEK293 cells by liquid scintillation spectrophotometry |
Bioorg Med Chem Lett 22: 3441-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.093
BindingDB Entry DOI: 10.7270/Q2W37XB1 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50009672
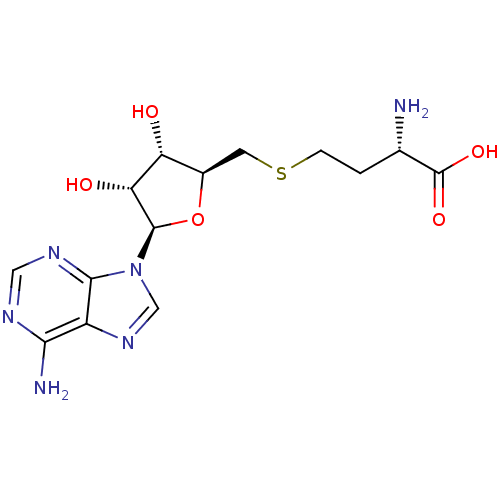
(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50434278
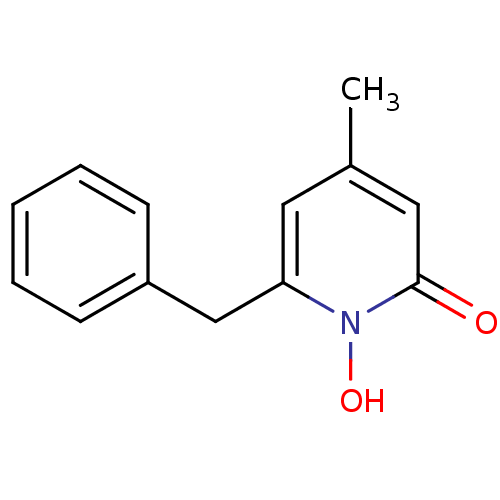
(CHEMBL2386126)Show InChI InChI=1S/C13H13NO2/c1-10-7-12(14(16)13(15)8-10)9-11-5-3-2-4-6-11/h2-8,16H,9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human IDH1 R132H mutant expressed in Escherichia coli BL21-CodonPlus assessed as reduction in NADPH consumption |
J Med Chem 57: 8307-18 (2014)
Article DOI: 10.1021/jm500660f
BindingDB Entry DOI: 10.7270/Q23J3FJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396990
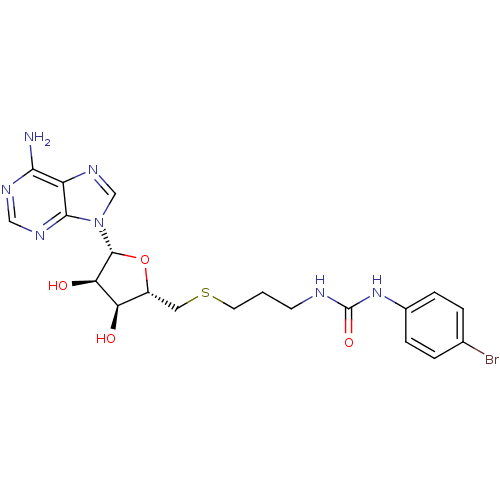
(CHEMBL2170995)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCCCNC(=O)Nc2ccc(Br)cc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24BrN7O4S/c21-11-2-4-12(5-3-11)27-20(31)23-6-1-7-33-8-13-15(29)16(30)19(32-13)28-10-26-14-17(22)24-9-25-18(14)28/h2-5,9-10,13,15-16,19,29-30H,1,6-8H2,(H2,22,24,25)(H2,23,27,31)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142240
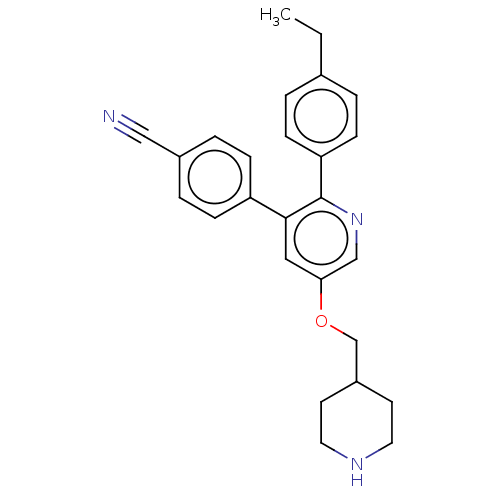
(CHEMBL3759861)Show SMILES CCc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C26H27N3O/c1-2-19-3-9-23(10-4-19)26-25(22-7-5-20(16-27)6-8-22)15-24(17-29-26)30-18-21-11-13-28-14-12-21/h3-10,15,17,21,28H,2,11-14,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50142249
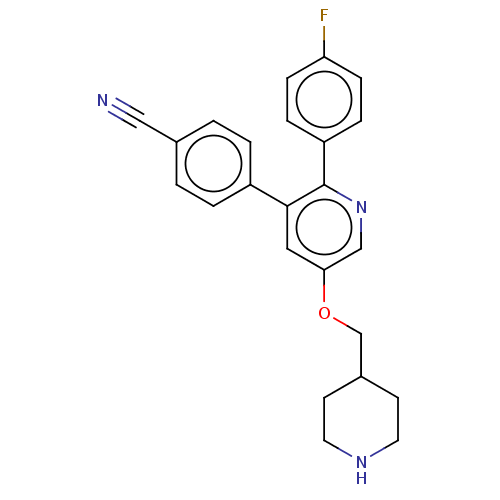
(CHEMBL3759939)Show SMILES Fc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1)C#N Show InChI InChI=1S/C24H22FN3O/c25-21-7-5-20(6-8-21)24-23(19-3-1-17(14-26)2-4-19)13-22(15-28-24)29-16-18-9-11-27-12-10-18/h1-8,13,15,18,27H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50139119
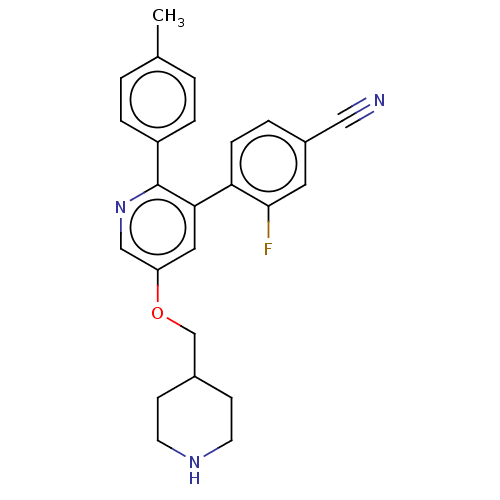
(CHEMBL3758849)Show SMILES Cc1ccc(cc1)-c1ncc(OCC2CCNCC2)cc1-c1ccc(cc1F)C#N Show InChI InChI=1S/C25H24FN3O/c1-17-2-5-20(6-3-17)25-23(22-7-4-19(14-27)12-24(22)26)13-21(15-29-25)30-16-18-8-10-28-11-9-18/h2-7,12-13,15,18,28H,8-11,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LSD1 catalytic domain (172 to 833 residues) using dimethylated H3K4 peptide substrate preincubated for 10 ... |
J Med Chem 59: 253-63 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01361
BindingDB Entry DOI: 10.7270/Q23J3FSW |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50029489
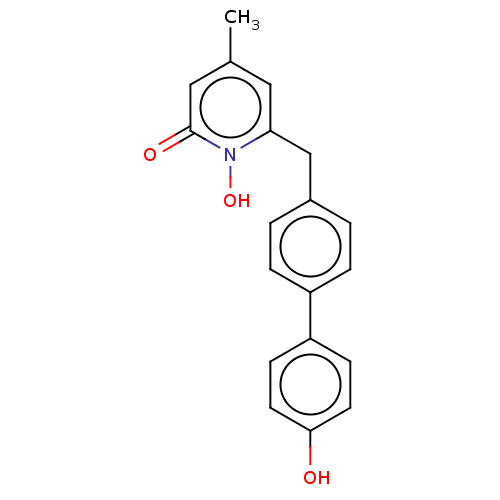
(CHEMBL3343463)Show InChI InChI=1S/C19H17NO3/c1-13-10-17(20(23)19(22)11-13)12-14-2-4-15(5-3-14)16-6-8-18(21)9-7-16/h2-11,21,23H,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human IDH1 R132H mutant expressed in Escherichia coli BL21-CodonPlus assessed as reduction in NADPH consumption |
J Med Chem 57: 8307-18 (2014)
Article DOI: 10.1021/jm500660f
BindingDB Entry DOI: 10.7270/Q23J3FJR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data