 Found 606 hits with Last Name = 'o''connor' and Initial = 'm'
Found 606 hits with Last Name = 'o''connor' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
J Nat Prod 60: 651-3 (1997)
Article DOI: 10.1021/np960644d
BindingDB Entry DOI: 10.7270/Q2ZC83R7 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM18771
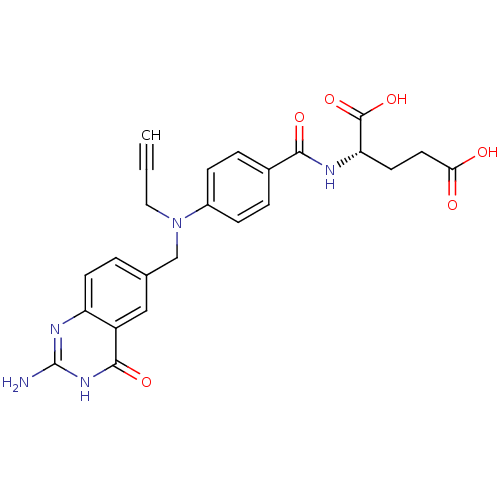
((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Binding affinity against Thymidylate synthase was measured in vitro |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50006687
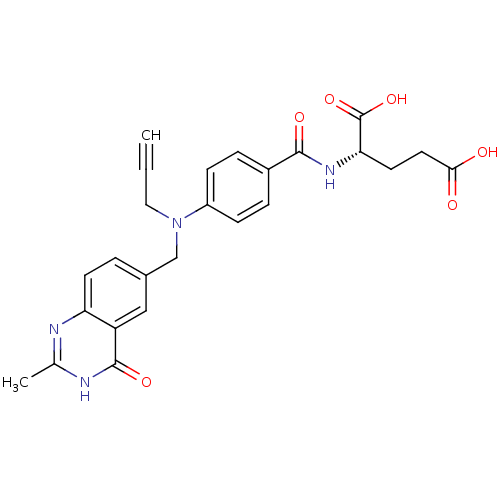
((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H24N4O6/c1-3-12-29(14-16-4-9-20-19(13-16)24(33)27-15(2)26-20)18-7-5-17(6-8-18)23(32)28-21(25(34)35)10-11-22(30)31/h1,4-9,13,21H,10-12,14H2,2H3,(H,28,32)(H,30,31)(H,34,35)(H,26,27,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Binding affinity against Thymidylate synthase was measured in vitro |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50014480
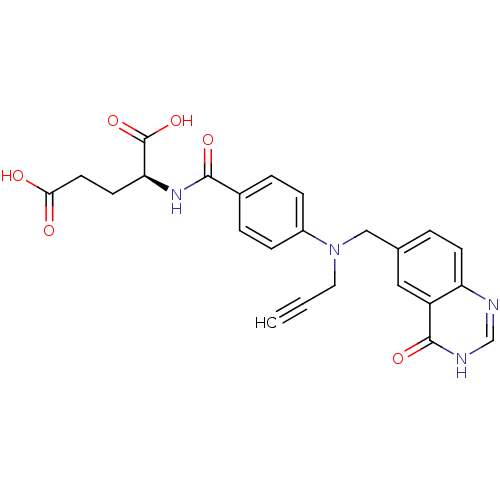
((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...)Show SMILES OC(=O)CC[C@H](NC(=O)c1ccc(cc1)N(CC#C)Cc1ccc2nc[nH]c(=O)c2c1)C(O)=O Show InChI InChI=1S/C24H22N4O6/c1-2-11-28(13-15-3-8-19-18(12-15)23(32)26-14-25-19)17-6-4-16(5-7-17)22(31)27-20(24(33)34)9-10-21(29)30/h1,3-8,12,14,20H,9-11,13H2,(H,27,31)(H,29,30)(H,33,34)(H,25,26,32)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Concentration required for in vitro inhibition of thymidylate synthase |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase in rat liver |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50014480

((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...)Show SMILES OC(=O)CC[C@H](NC(=O)c1ccc(cc1)N(CC#C)Cc1ccc2nc[nH]c(=O)c2c1)C(O)=O Show InChI InChI=1S/C24H22N4O6/c1-2-11-28(13-15-3-8-19-18(12-15)23(32)26-14-25-19)17-6-4-16(5-7-17)22(31)27-20(24(33)34)9-10-21(29)30/h1,3-8,12,14,20H,9-11,13H2,(H,27,31)(H,29,30)(H,33,34)(H,25,26,32)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase in rat liver |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50006687

((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H24N4O6/c1-3-12-29(14-16-4-9-20-19(13-16)24(33)27-15(2)26-20)18-7-5-17(6-8-18)23(32)28-21(25(34)35)10-11-22(30)31/h1,4-9,13,21H,10-12,14H2,2H3,(H,28,32)(H,30,31)(H,34,35)(H,26,27,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase in rat liver |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50246936
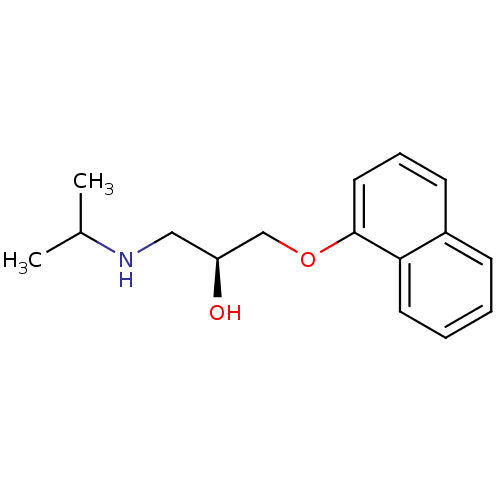
((-)-(S)-Propranolol | 1-(ISOPROPYLAMINO)-3-(1-NAPH...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
J Nat Prod 60: 651-3 (1997)
Article DOI: 10.1021/np960644d
BindingDB Entry DOI: 10.7270/Q2ZC83R7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194429
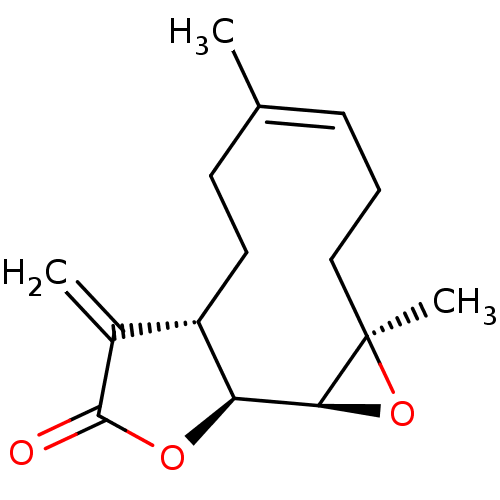
((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...)Show SMILES C\C1=C\CC[C@@]2(C)O[C@H]2[C@H]2OC(=O)C(=C)[C@@H]2CC1 |r,t:1| Show InChI InChI=1S/C15H20O3/c1-9-5-4-8-15(3)13(18-15)12-11(7-6-9)10(2)14(16)17-12/h5,11-13H,2,4,6-8H2,1,3H3/b9-5-/t11-,12-,13-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
J Nat Prod 60: 651-3 (1997)
Article DOI: 10.1021/np960644d
BindingDB Entry DOI: 10.7270/Q2ZC83R7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194429

((-)-parthenolide | (1aR,7aS,10aS,10bS,Z)-1a,5-dime...)Show SMILES C\C1=C\CC[C@@]2(C)O[C@H]2[C@H]2OC(=O)C(=C)[C@@H]2CC1 |r,t:1| Show InChI InChI=1S/C15H20O3/c1-9-5-4-8-15(3)13(18-15)12-11(7-6-9)10(2)14(16)17-12/h5,11-13H,2,4,6-8H2,1,3H3/b9-5-/t11-,12-,13-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in rat brain membrane |
J Nat Prod 60: 651-3 (1997)
Article DOI: 10.1021/np960644d
BindingDB Entry DOI: 10.7270/Q2ZC83R7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183224
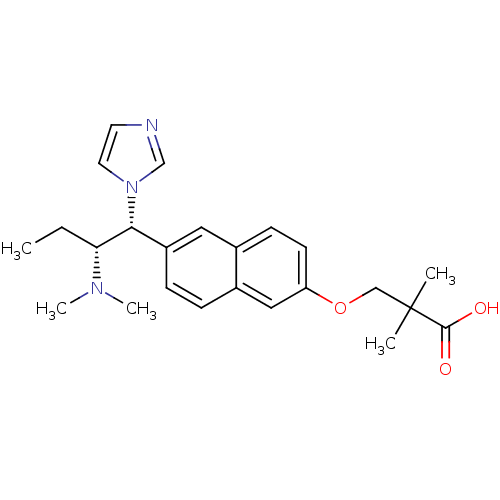
(3-[6-(2-dimethylamino-1-imidazol-1-yl-butyl)-napht...)Show SMILES CC[C@H]([C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C24H31N3O3/c1-6-21(26(4)5)22(27-12-11-25-16-27)19-8-7-18-14-20(10-9-17(18)13-19)30-15-24(2,3)23(28)29/h7-14,16,21-22H,6,15H2,1-5H3,(H,28,29)/t21-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183243

(1-((6-((1R,2R)-2-(diethylamino)-1-(1H-imidazol-1-y...)Show SMILES CCN(CC)[C@H](C)[C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C27H35N3O3/c1-4-29(5-2)20(3)25(30-15-14-28-19-30)23-9-8-22-17-24(11-10-21(22)16-23)33-18-27(26(31)32)12-6-7-13-27/h8-11,14-17,19-20,25H,4-7,12-13,18H2,1-3H3,(H,31,32)/t20-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183229
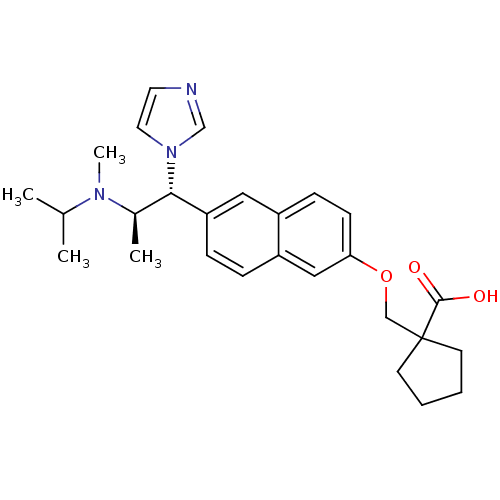
(1-((6-((1R,2R)-1-(1H-imidazol-1-yl)-2-(isopropyl(m...)Show SMILES CC(C)N(C)[C@H](C)[C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C27H35N3O3/c1-19(2)29(4)20(3)25(30-14-13-28-18-30)23-8-7-22-16-24(10-9-21(22)15-23)33-17-27(26(31)32)11-5-6-12-27/h7-10,13-16,18-20,25H,5-6,11-12,17H2,1-4H3,(H,31,32)/t20-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336325
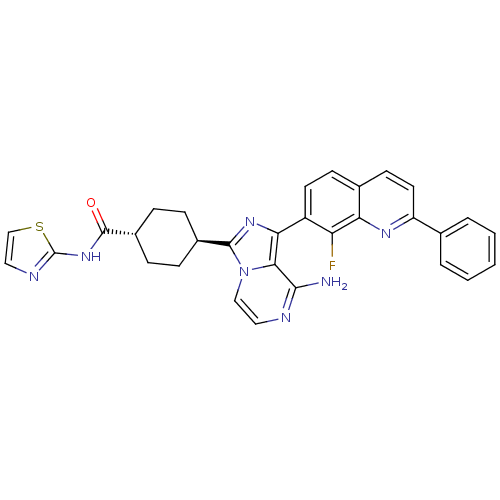
(CHEMBL1667943 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nccs1 |r,wU:27.31,wD:30.38,(5.48,2.11,;5.49,.57,;4.16,-.2,;4.16,-1.75,;5.49,-2.52,;6.83,-1.74,;8.3,-2.21,;9.2,-.95,;8.29,.29,;9.06,1.62,;8.29,2.94,;9.05,4.28,;10.6,4.28,;11.36,5.6,;12.89,5.61,;13.67,4.27,;12.9,2.95,;11.37,2.95,;10.6,1.61,;11.37,.28,;15.2,4.27,;15.97,2.94,;17.51,2.94,;18.28,4.27,;17.5,5.61,;15.96,5.6,;6.82,-.2,;8.79,-3.67,;10.29,-3.97,;10.77,-5.44,;9.74,-6.59,;8.24,-6.27,;7.76,-4.81,;10.22,-8.05,;9.44,-9.37,;11.76,-8.07,;12.52,-9.41,;12.05,-10.87,;13.3,-11.77,;14.54,-10.87,;14.07,-9.4,)| Show InChI InChI=1S/C31H26FN7OS/c32-24-22(12-10-19-11-13-23(36-25(19)24)18-4-2-1-3-5-18)26-27-28(33)34-14-16-39(27)29(37-26)20-6-8-21(9-7-20)30(40)38-31-35-15-17-41-31/h1-5,10-17,20-21H,6-9H2,(H2,33,34)(H,35,38,40)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553940
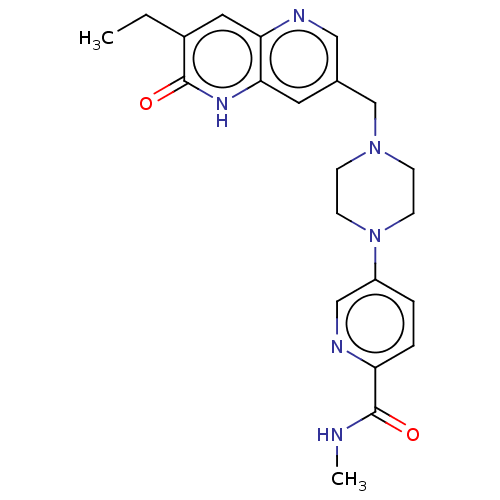
(US11325906, Example 4)Show SMILES CCc1cc2ncc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553937
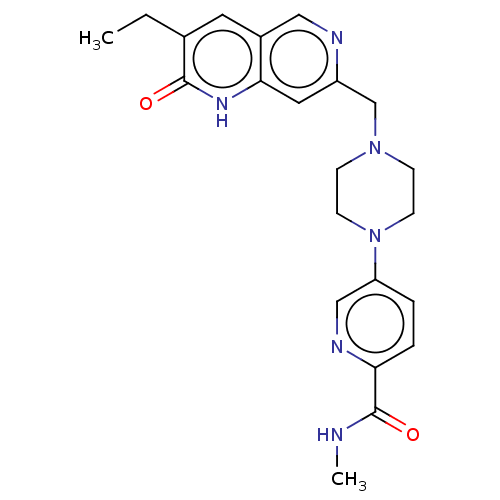
(US11325906, Example 1)Show SMILES CCc1cc2cnc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183237
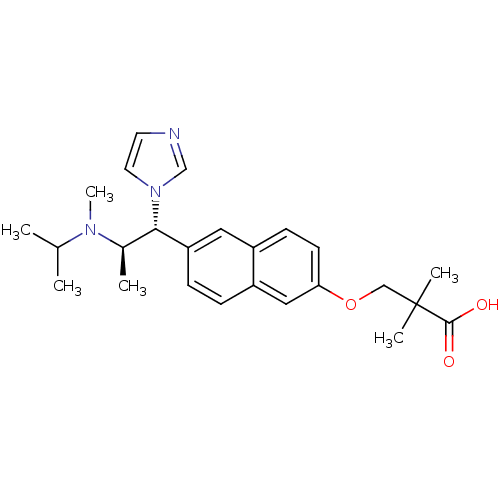
(3-(6-((1R,2R)-1-(1H-imidazol-1-yl)-2-(isopropyl(me...)Show SMILES CC(C)N(C)[C@H](C)[C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C25H33N3O3/c1-17(2)27(6)18(3)23(28-12-11-26-16-28)21-8-7-20-14-22(10-9-19(20)13-21)31-15-25(4,5)24(29)30/h7-14,16-18,23H,15H2,1-6H3,(H,29,30)/t18-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336329
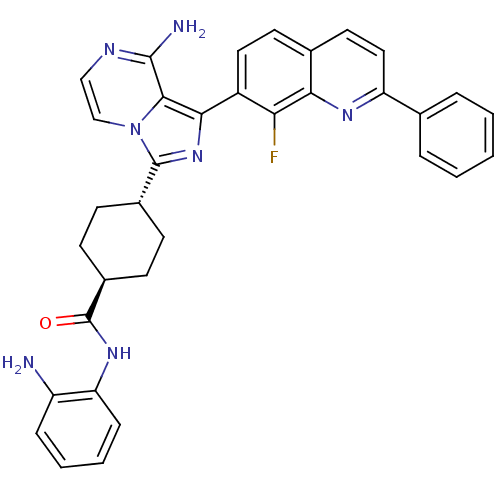
(CHEMBL1667947 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1ccccc1NC(=O)[C@H]1CC[C@@H](CC1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:13.17,wD:10.10,(28.81,-26.74,;28.05,-25.39,;28.84,-24.07,;28.08,-22.73,;26.54,-22.71,;25.76,-24.04,;26.52,-25.37,;25.73,-26.69,;24.19,-26.68,;23.41,-28,;23.71,-25.21,;24.74,-24.06,;24.26,-22.6,;22.75,-22.3,;21.73,-23.44,;22.21,-24.89,;22.27,-20.83,;23.17,-19.58,;22.26,-18.34,;23.03,-17.01,;22.26,-15.69,;23.02,-14.35,;24.57,-14.35,;25.33,-13.03,;26.86,-13.02,;27.63,-14.35,;26.87,-15.68,;25.33,-15.68,;24.57,-17.02,;25.34,-18.35,;29.17,-14.35,;29.94,-15.69,;31.48,-15.69,;32.25,-14.36,;31.47,-13.02,;29.93,-13.02,;20.79,-18.82,;19.46,-18.06,;19.45,-16.52,;18.13,-18.83,;18.13,-20.37,;19.46,-21.15,;20.8,-20.37,)| Show InChI InChI=1S/C34H30FN7O/c35-28-24(16-14-21-15-17-26(39-29(21)28)20-6-2-1-3-7-20)30-31-32(37)38-18-19-42(31)33(41-30)22-10-12-23(13-11-22)34(43)40-27-9-5-4-8-25(27)36/h1-9,14-19,22-23H,10-13,36H2,(H2,37,38)(H,40,43)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601780
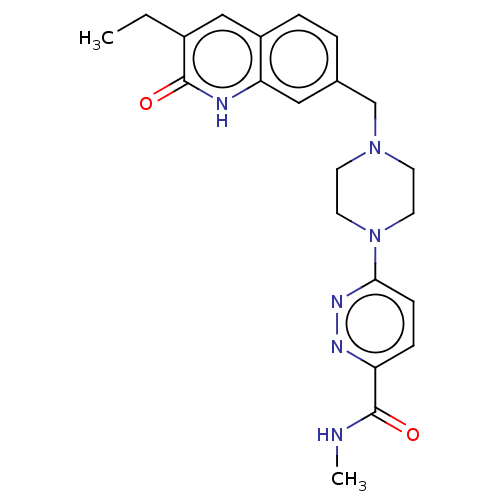
(CHEMBL5202060)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(nn3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183231

(1-((6-((1R,2R)-2-(ethyl(methyl)amino)-1-(1H-imidaz...)Show SMILES CCN(C)[C@H](C)[C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C26H33N3O3/c1-4-28(3)19(2)24(29-14-13-27-18-29)22-8-7-21-16-23(10-9-20(21)15-22)32-17-26(25(30)31)11-5-6-12-26/h7-10,13-16,18-19,24H,4-6,11-12,17H2,1-3H3,(H,30,31)/t19-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50033933

((R)-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazolin...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](C(O)=O)c4cccc(c4)[N+]([O-])=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C29H24FN5O6/c1-4-10-34(15-19-13-23-25(11-16(19)2)31-17(3)32-28(23)37)20-8-9-22(24(30)14-20)27(36)33-26(29(38)39)18-6-5-7-21(12-18)35(40)41/h1,5-9,11-14,26H,10,15H2,2-3H3,(H,33,36)(H,38,39)(H,31,32,37)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) |
J Med Chem 38: 994-1004 (1995)
BindingDB Entry DOI: 10.7270/Q2J67FZ7 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336327
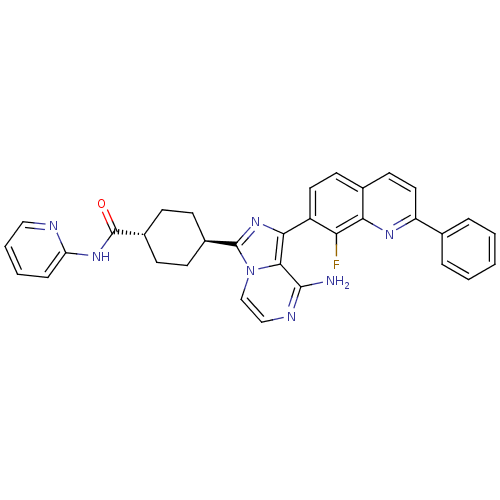
(CHEMBL1667945 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1ccccn1 |r,wU:27.31,wD:30.38,(-8.63,-16.09,;-8.62,-17.63,;-9.95,-18.4,;-9.95,-19.94,;-8.62,-20.71,;-7.28,-19.93,;-5.81,-20.4,;-4.91,-19.15,;-5.82,-17.91,;-5.05,-16.58,;-5.82,-15.25,;-5.06,-13.92,;-3.51,-13.92,;-2.75,-12.59,;-1.22,-12.59,;-.44,-13.92,;-1.21,-15.25,;-2.74,-15.25,;-3.51,-16.58,;-2.74,-17.92,;1.09,-13.92,;1.86,-15.26,;3.4,-15.26,;4.17,-13.92,;3.39,-12.58,;1.85,-12.59,;-7.29,-18.39,;-5.32,-21.86,;-3.82,-22.16,;-3.34,-23.63,;-4.37,-24.78,;-5.87,-24.46,;-6.35,-23.01,;-3.89,-26.25,;-4.67,-27.57,;-2.35,-26.26,;-1.56,-24.94,;-.02,-24.96,;.76,-23.64,;.01,-22.29,;-1.54,-22.28,;-2.32,-23.61,)| Show InChI InChI=1S/C33H28FN7O/c34-27-24(15-13-21-14-16-25(38-28(21)27)20-6-2-1-3-7-20)29-30-31(35)37-18-19-41(30)32(40-29)22-9-11-23(12-10-22)33(42)39-26-8-4-5-17-36-26/h1-8,13-19,22-23H,9-12H2,(H2,35,37)(H,36,39,42)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183225
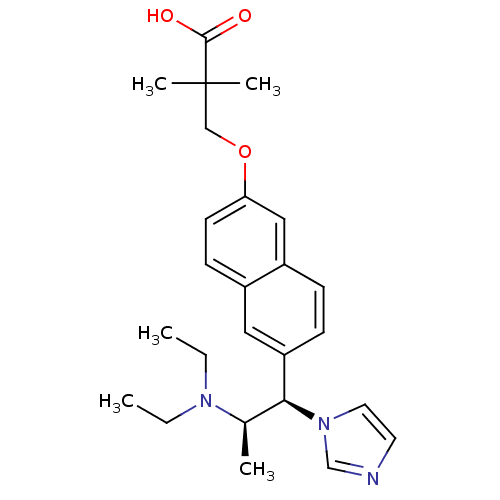
(3-(6-((1R,2R)-2-(diethylamino)-1-(1H-imidazol-1-yl...)Show SMILES CCN(CC)[C@H](C)[C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C25H33N3O3/c1-6-27(7-2)18(3)23(28-13-12-26-17-28)21-9-8-20-15-22(11-10-19(20)14-21)31-16-25(4,5)24(29)30/h8-15,17-18,23H,6-7,16H2,1-5H3,(H,29,30)/t18-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336324
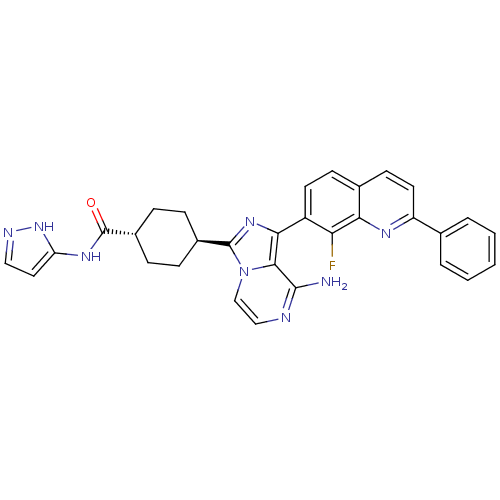
(CHEMBL1667942 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1ccn[nH]1 |r,wU:27.31,wD:30.38,(-8.21,2.51,;-8.21,.97,;-9.54,.19,;-9.54,-1.35,;-8.21,-2.12,;-6.87,-1.34,;-5.4,-1.81,;-4.5,-.56,;-5.41,.69,;-4.64,2.01,;-5.41,3.34,;-4.64,4.67,;-3.1,4.68,;-2.33,6,;-.8,6,;-.03,4.67,;-.8,3.34,;-2.33,3.34,;-3.1,2.01,;-2.33,.67,;1.5,4.67,;2.27,3.33,;3.81,3.33,;4.58,4.67,;3.8,6.01,;2.27,6,;-6.87,.2,;-4.91,-3.27,;-3.4,-3.57,;-2.93,-5.04,;-3.95,-6.19,;-5.46,-5.87,;-5.94,-4.41,;-3.47,-7.65,;-4.26,-8.98,;-1.93,-7.67,;-1.18,-9.01,;.37,-9.01,;.85,-10.47,;-.4,-11.38,;-1.65,-10.47,)| Show InChI InChI=1S/C31H27FN8O/c32-25-22(12-10-19-11-13-23(36-26(19)25)18-4-2-1-3-5-18)27-28-29(33)34-16-17-40(28)30(38-27)20-6-8-21(9-7-20)31(41)37-24-14-15-35-39-24/h1-5,10-17,20-21H,6-9H2,(H2,33,34)(H2,35,37,39,41)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336323

(CHEMBL1667941 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nncs1 |r,wU:27.31,wD:30.38,(36.37,-27.21,;36.37,-28.75,;35.04,-29.52,;35.04,-31.06,;36.38,-31.83,;37.72,-31.05,;39.19,-31.52,;40.09,-30.27,;39.17,-29.02,;39.94,-27.7,;39.17,-26.37,;39.94,-25.04,;41.49,-25.04,;42.25,-23.71,;43.78,-23.71,;44.55,-25.04,;43.78,-26.37,;42.25,-26.37,;41.48,-27.7,;42.25,-29.04,;46.09,-25.04,;46.85,-26.38,;48.39,-26.38,;49.16,-25.04,;48.39,-23.7,;46.85,-23.71,;37.71,-29.51,;39.67,-32.98,;41.18,-33.28,;41.66,-34.75,;40.63,-35.9,;39.12,-35.58,;38.64,-34.12,;41.11,-37.36,;40.32,-38.69,;42.65,-37.38,;43.4,-38.72,;44.94,-38.9,;45.24,-40.41,;43.9,-41.16,;42.77,-40.12,)| Show InChI InChI=1S/C30H25FN8OS/c31-23-21(12-10-18-11-13-22(35-24(18)23)17-4-2-1-3-5-17)25-26-27(32)33-14-15-39(26)28(36-25)19-6-8-20(9-7-19)29(40)37-30-38-34-16-41-30/h1-5,10-16,19-20H,6-9H2,(H2,32,33)(H,37,38,40)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336321
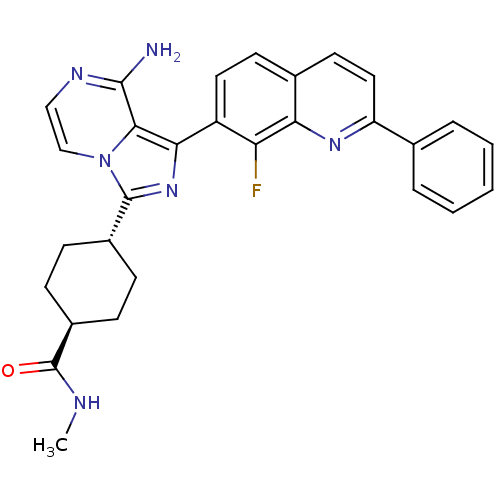
(CHEMBL1667939 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES CNC(=O)[C@H]1CC[C@@H](CC1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:7.10,wD:4.3,(12.87,-39.64,;12.08,-40.96,;10.54,-40.95,;9.76,-42.27,;10.06,-39.48,;11.09,-38.33,;10.61,-36.86,;9.11,-36.56,;8.08,-37.71,;8.56,-39.16,;8.62,-35.1,;9.52,-33.85,;8.61,-32.61,;9.38,-31.28,;8.61,-29.95,;9.37,-28.62,;10.92,-28.62,;11.68,-27.29,;13.21,-27.29,;13.99,-28.62,;13.22,-29.95,;11.69,-29.95,;10.92,-31.28,;11.69,-32.62,;15.52,-28.62,;16.29,-29.96,;17.83,-29.96,;18.6,-28.62,;17.82,-27.29,;16.28,-27.29,;7.14,-33.09,;5.81,-32.33,;5.8,-30.79,;4.48,-33.1,;4.48,-34.64,;5.81,-35.41,;7.15,-34.63,)| Show InChI InChI=1S/C29H27FN6O/c1-32-29(37)20-9-7-19(8-10-20)28-35-25(26-27(31)33-15-16-36(26)28)21-13-11-18-12-14-22(34-24(18)23(21)30)17-5-3-2-4-6-17/h2-6,11-16,19-20H,7-10H2,1H3,(H2,31,33)(H,32,37)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553947
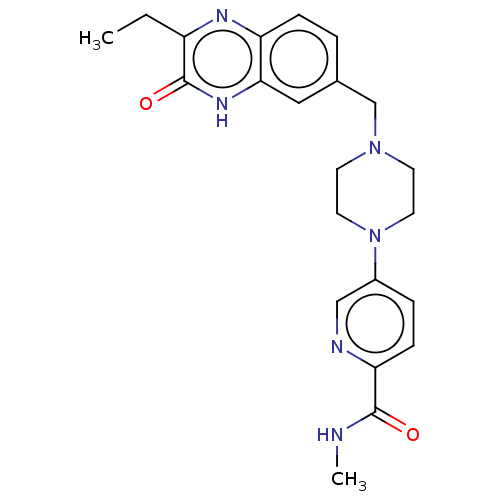
(US11325906, Example 11)Show SMILES CCc1nc2ccc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601781
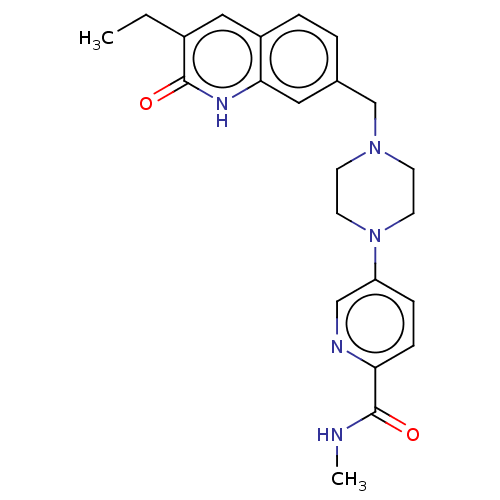
(CHEMBL5197101)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Mus musculus) | BDBM50033920
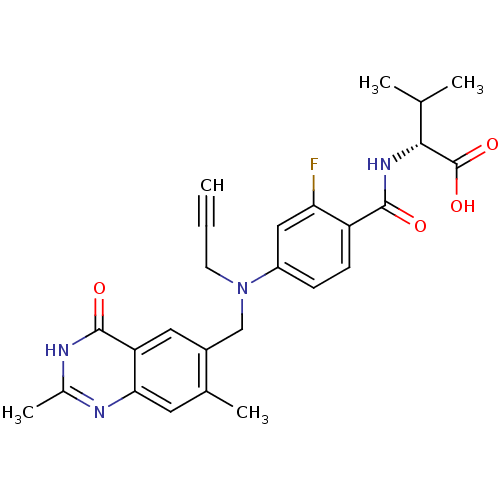
((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES CC(C)[C@@H](NC(=O)c1ccc(cc1F)N(CC#C)Cc1cc2c(cc1C)nc(C)[nH]c2=O)C(O)=O Show InChI InChI=1S/C26H27FN4O4/c1-6-9-31(13-17-11-20-22(10-15(17)4)28-16(5)29-25(20)33)18-7-8-19(21(27)12-18)24(32)30-23(14(2)3)26(34)35/h1,7-8,10-12,14,23H,9,13H2,2-5H3,(H,30,32)(H,34,35)(H,28,29,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) |
J Med Chem 38: 994-1004 (1995)
BindingDB Entry DOI: 10.7270/Q2J67FZ7 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336322
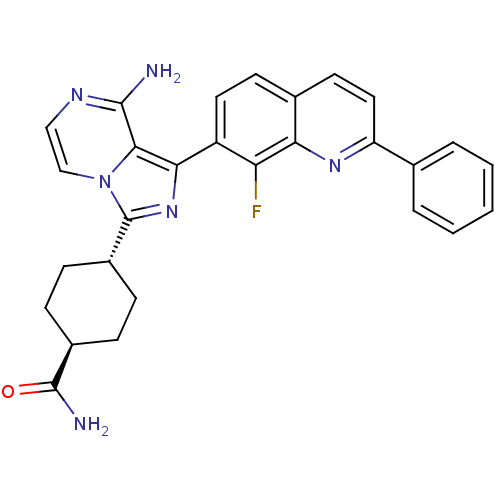
(CHEMBL1667940 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES NC(=O)[C@H]1CC[C@@H](CC1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)nccn12 |r,wU:6.9,wD:3.2,(26.07,-41.48,;24.53,-41.46,;23.74,-42.79,;24.05,-40,;25.07,-38.85,;24.6,-37.38,;23.09,-37.08,;22.06,-38.22,;22.54,-39.68,;22.6,-35.62,;23.5,-34.37,;22.59,-33.12,;23.36,-31.8,;22.59,-30.47,;23.36,-29.14,;24.9,-29.13,;25.67,-27.81,;27.2,-27.81,;27.97,-29.14,;27.2,-30.47,;25.67,-30.47,;24.9,-31.8,;25.67,-33.14,;29.5,-29.14,;30.27,-30.48,;31.81,-30.48,;32.58,-29.14,;31.8,-27.8,;30.27,-27.81,;21.13,-33.61,;19.79,-32.84,;19.79,-31.3,;18.46,-33.62,;18.46,-35.16,;19.79,-35.93,;21.13,-35.15,)| Show InChI InChI=1S/C28H25FN6O/c29-22-20(12-10-17-11-13-21(33-23(17)22)16-4-2-1-3-5-16)24-25-26(30)32-14-15-35(25)28(34-24)19-8-6-18(7-9-19)27(31)36/h1-5,10-15,18-19H,6-9H2,(H2,30,32)(H2,31,36)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566
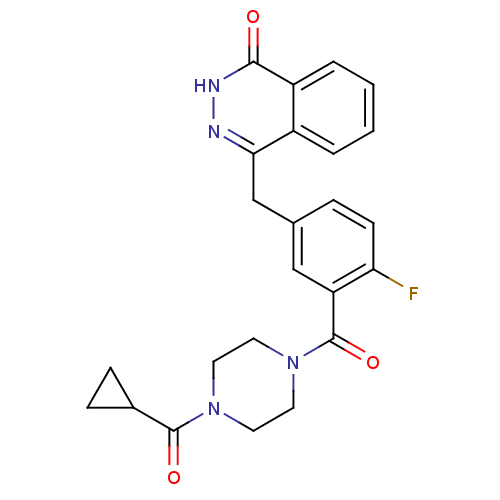
(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336315
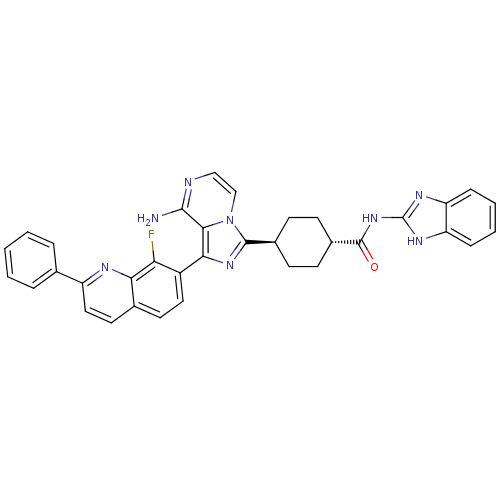
(CHEMBL1667948 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccccc2[nH]1 |r,wU:27.31,wD:30.38,(-9.22,-32.11,;-9.21,-33.66,;-10.54,-34.43,;-10.55,-35.97,;-9.21,-36.74,;-7.87,-35.96,;-6.4,-36.43,;-5.5,-35.18,;-6.41,-33.93,;-5.64,-32.61,;-6.41,-31.28,;-5.64,-29.95,;-4.09,-29.94,;-3.33,-28.62,;-1.8,-28.61,;-1.03,-29.95,;-1.8,-31.28,;-3.33,-31.27,;-4.1,-32.61,;-3.33,-33.95,;.51,-29.95,;1.28,-31.28,;2.82,-31.28,;3.59,-29.95,;2.81,-28.61,;1.27,-28.62,;-7.88,-34.42,;-5.91,-37.9,;-4.4,-38.2,;-3.92,-39.67,;-4.95,-40.82,;-6.46,-40.5,;-6.94,-39.04,;-4.47,-42.28,;-5.26,-43.61,;-2.93,-42.3,;-2.15,-40.97,;-2.75,-39.55,;-1.6,-38.53,;-1.58,-37,;-.25,-36.25,;1.08,-37.03,;1.06,-38.56,;-.27,-39.31,;-.61,-40.82,)| Show InChI InChI=1S/C35H29FN8O/c36-28-24(16-14-21-15-17-25(39-29(21)28)20-6-2-1-3-7-20)30-31-32(37)38-18-19-44(31)33(42-30)22-10-12-23(13-11-22)34(45)43-35-40-26-8-4-5-9-27(26)41-35/h1-9,14-19,22-23H,10-13H2,(H2,37,38)(H2,40,41,43,45)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50033901
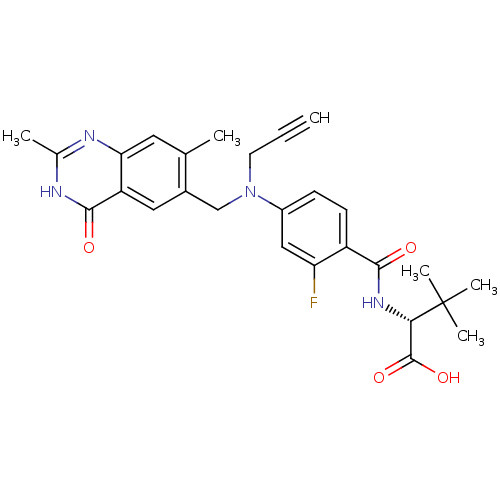
((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@@H](C(O)=O)C(C)(C)C)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C27H29FN4O4/c1-7-10-32(14-17-12-20-22(11-15(17)2)29-16(3)30-25(20)34)18-8-9-19(21(28)13-18)24(33)31-23(26(35)36)27(4,5)6/h1,8-9,11-13,23H,10,14H2,2-6H3,(H,31,33)(H,35,36)(H,29,30,34)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Ability to inhibit Thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) |
J Med Chem 38: 994-1004 (1995)
BindingDB Entry DOI: 10.7270/Q2J67FZ7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601767
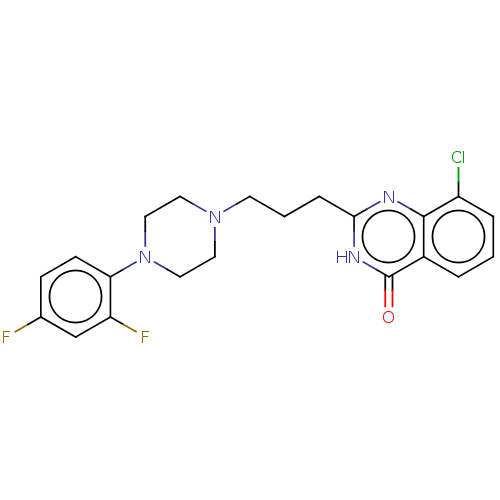
(CHEMBL5190481)Show SMILES Fc1ccc(N2CCN(CCCc3nc4c(Cl)cccc4c(=O)[nH]3)CC2)c(F)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130
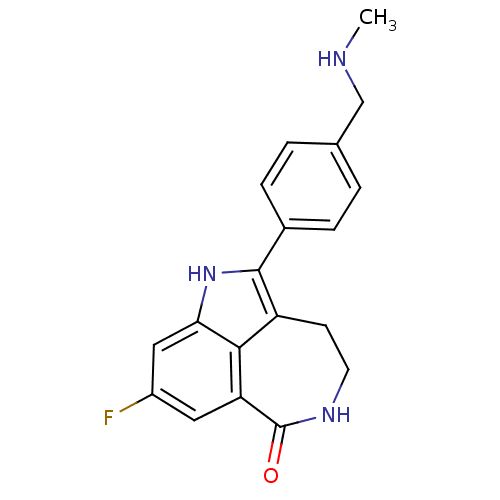
(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 26A1
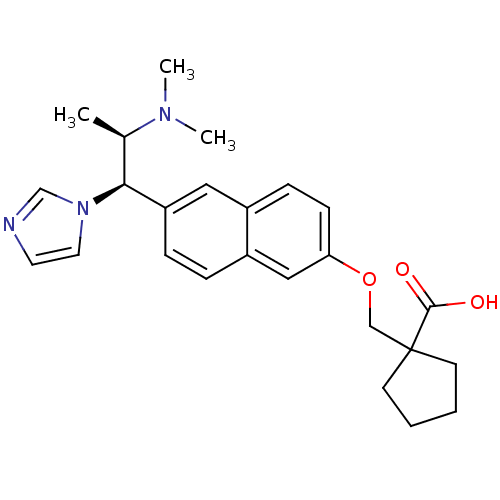
(Homo sapiens (Human)) | BDBM50183228
(1-((6-((1R,2R)-2-(dimethylamino)-1-(1H-imidazol-1-...)Show SMILES C[C@H]([C@@H](c1ccc2cc(OCC3(CCCC3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C25H31N3O3/c1-18(27(2)3)23(28-13-12-26-17-28)21-7-6-20-15-22(9-8-19(20)14-21)31-16-25(24(29)30)10-4-5-11-25/h6-9,12-15,17-18,23H,4-5,10-11,16H2,1-3H3,(H,29,30)/t18-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601776
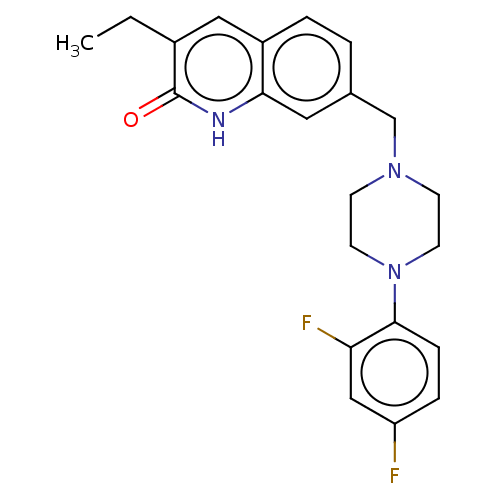
(CHEMBL5196187)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(F)cc3F)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336328
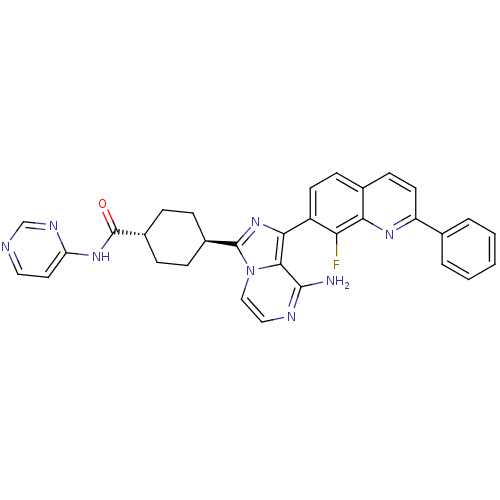
(CHEMBL1667946 | trans-4-(8-amino-1-(8-fluoro-2-phe...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1ccncn1 |r,wU:27.31,wD:30.38,(5.26,-16.47,;5.26,-18.01,;3.93,-18.78,;3.93,-20.33,;5.27,-21.1,;6.61,-20.32,;8.08,-20.79,;8.98,-19.53,;8.06,-18.29,;8.83,-16.97,;8.07,-15.64,;8.83,-14.3,;10.38,-14.3,;11.14,-12.98,;12.67,-12.97,;13.45,-14.31,;12.68,-15.63,;11.14,-15.63,;10.38,-16.97,;11.15,-18.3,;14.98,-14.31,;15.75,-15.64,;17.29,-15.64,;18.06,-14.31,;17.28,-12.97,;15.74,-12.98,;6.6,-18.78,;8.56,-22.25,;10.07,-22.55,;10.55,-24.02,;9.52,-25.17,;8.01,-24.85,;7.54,-23.39,;10,-26.63,;9.22,-27.96,;11.54,-26.65,;12.33,-25.32,;13.87,-25.35,;14.65,-24.03,;13.89,-22.68,;12.35,-22.67,;11.57,-23.99,)| Show InChI InChI=1S/C32H27FN8O/c33-26-23(12-10-20-11-13-24(38-27(20)26)19-4-2-1-3-5-19)28-29-30(34)36-16-17-41(29)31(40-28)21-6-8-22(9-7-21)32(42)39-25-14-15-35-18-37-25/h1-5,10-18,21-22H,6-9H2,(H2,34,36)(H,35,37,39,42)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601779
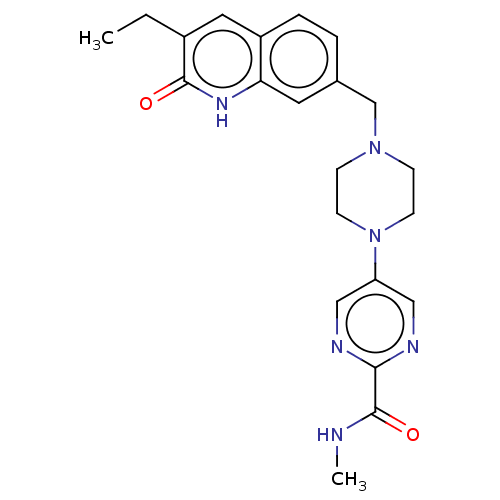
(CHEMBL5183769)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3cnc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336318
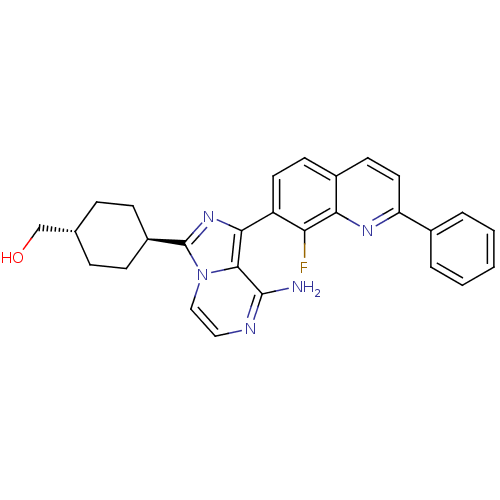
(CHEMBL1667936 | trans-2-(3-(-4-ethylcyclohexyl)-7-...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@H](CO)CC1 |r,wU:27.31,wD:30.35,(3.1,-15.73,;3.11,-17.27,;1.78,-18.04,;1.78,-19.59,;3.11,-20.36,;4.45,-19.58,;5.92,-20.05,;6.82,-18.8,;5.91,-17.55,;6.68,-16.23,;5.91,-14.9,;6.67,-13.57,;8.22,-13.56,;8.98,-12.24,;10.51,-12.24,;11.29,-13.57,;10.52,-14.9,;8.99,-14.89,;8.22,-16.23,;8.99,-17.57,;12.82,-13.57,;13.59,-14.91,;15.13,-14.91,;15.9,-13.57,;15.12,-12.23,;13.58,-12.24,;4.44,-18.04,;6.41,-21.51,;7.91,-21.81,;8.39,-23.28,;7.36,-24.43,;7.84,-25.89,;9.35,-26.21,;5.86,-24.11,;5.38,-22.65,)| Show InChI InChI=1S/C28H26FN5O/c29-23-21(12-10-19-11-13-22(32-24(19)23)18-4-2-1-3-5-18)25-26-27(30)31-14-15-34(26)28(33-25)20-8-6-17(16-35)7-9-20/h1-5,10-15,17,20,35H,6-9,16H2,(H2,30,31)/t17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R expressed in mouse 3T3 cells by ELISA based assay |
Bioorg Med Chem Lett 21: 1176-80 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.094
BindingDB Entry DOI: 10.7270/Q2J103DJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621
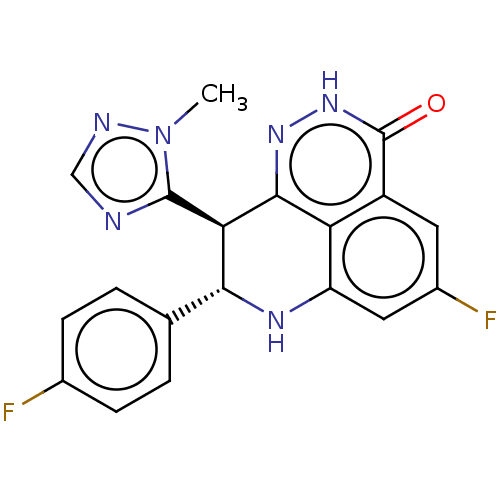
(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601768
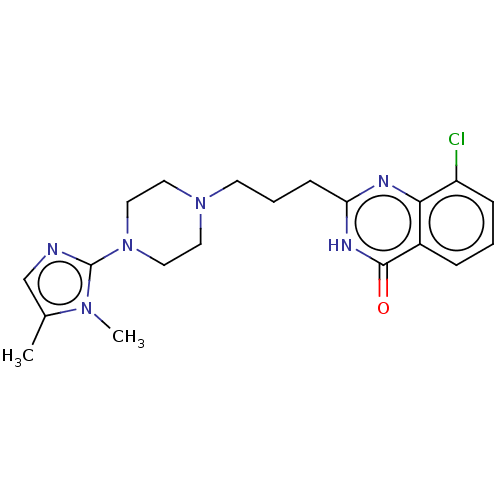
(CHEMBL5193246)Show SMILES Cc1cnc(N2CCN(CCCc3nc4c(Cl)cccc4c(=O)[nH]3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Mus musculus) | BDBM50033913
((R)-2-{4-[(2,7-Dimethyl-4-oxo-3,4-dihydro-quinazol...)Show SMILES Cc1nc2cc(C)c(CN(CC#C)c3ccc(C(=O)N[C@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H25FN4O6/c1-4-9-31(13-16-11-19-22(10-14(16)2)28-15(3)29-25(19)35)17-5-6-18(20(27)12-17)24(34)30-21(26(36)37)7-8-23(32)33/h1,5-6,10-12,21H,7-9,13H2,2-3H3,(H,30,34)(H,32,33)(H,36,37)(H,28,29,35)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase purified from mouse L1210 leukemia cells that overproduce thymidylate synthase (TS) |
J Med Chem 38: 994-1004 (1995)
BindingDB Entry DOI: 10.7270/Q2J67FZ7 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601769
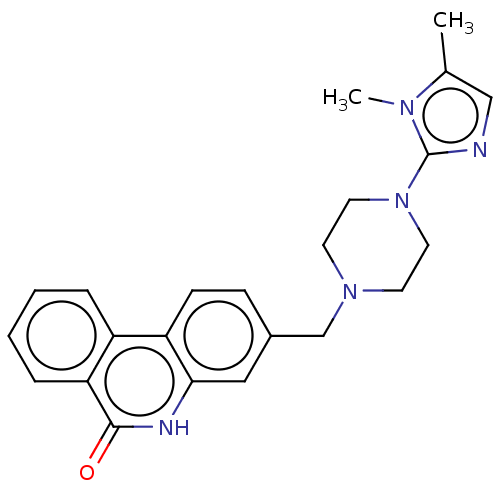
(CHEMBL5191396)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c(c3)[nH]c(=O)c3ccccc43)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
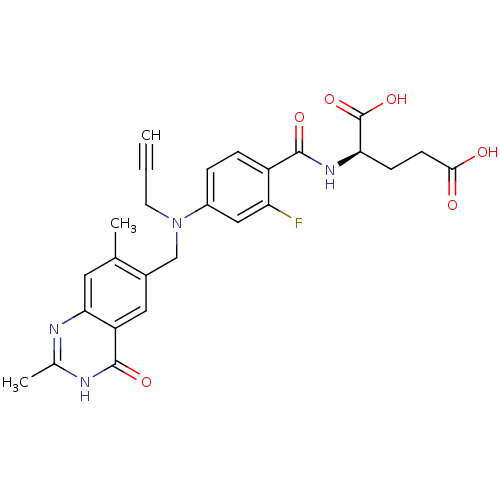
Thymidylate synthase
(Mus musculus) | BDBM50014498
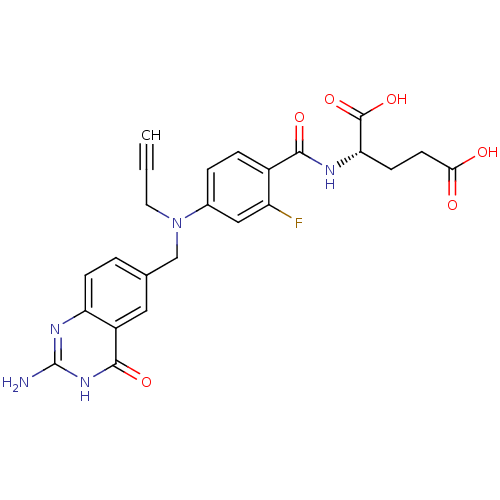
((S)-2-(4-(((2-amino-4-oxo-3,4-dihydroquinazolin-6-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H22FN5O6/c1-2-9-30(12-13-3-6-18-16(10-13)22(34)29-24(26)28-18)14-4-5-15(17(25)11-14)21(33)27-19(23(35)36)7-8-20(31)32/h1,3-6,10-11,19H,7-9,12H2,(H,27,33)(H,31,32)(H,35,36)(H3,26,28,29,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined |
J Med Chem 33: 3067-71 (1990)
BindingDB Entry DOI: 10.7270/Q2V40T5X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
(Homo sapiens (Human)) | BDBM50183241

(3-(6-((1R,2R)-2-(ethyl(methyl)amino)-1-(1H-imidazo...)Show SMILES CCN(C)[C@H](C)[C@@H](c1ccc2cc(OCC(C)(C)C(O)=O)ccc2c1)n1ccnc1 Show InChI InChI=1S/C24H31N3O3/c1-6-26(5)17(2)22(27-12-11-25-16-27)20-8-7-19-14-21(10-9-18(19)13-20)30-15-24(3,4)23(28)29/h7-14,16-17,22H,6,15H2,1-5H3,(H,28,29)/t17-,22+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601772
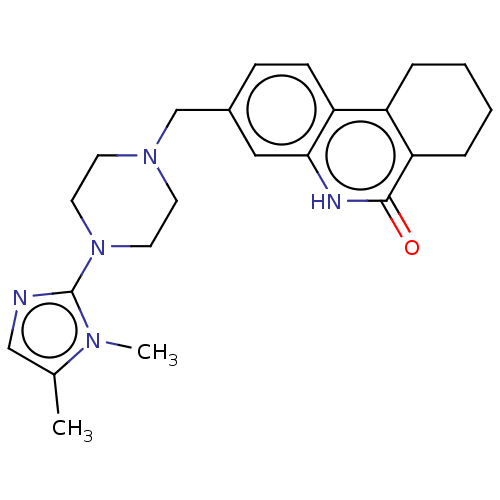
(CHEMBL5177596)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c5CCCCc5c(=O)[nH]c4c3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 26A1
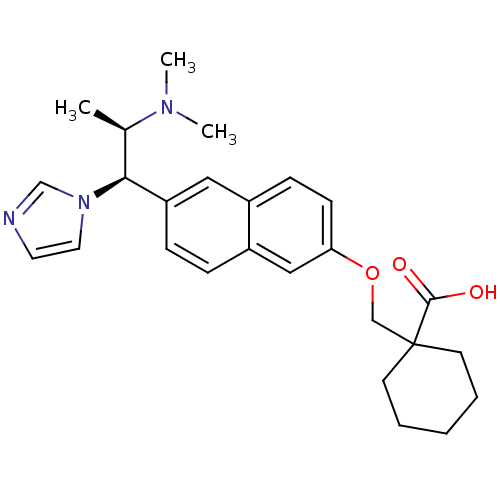
(Homo sapiens (Human)) | BDBM50183230
(1-((6-((1R,2R)-2-(dimethylamino)-1-(1H-imidazol-1-...)Show SMILES C[C@H]([C@@H](c1ccc2cc(OCC3(CCCCC3)C(O)=O)ccc2c1)n1ccnc1)N(C)C Show InChI InChI=1S/C26H33N3O3/c1-19(28(2)3)24(29-14-13-27-18-29)22-8-7-21-16-23(10-9-20(21)15-22)32-17-26(25(30)31)11-5-4-6-12-26/h7-10,13-16,18-19,24H,4-6,11-12,17H2,1-3H3,(H,30,31)/t19-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP26 expressed in human T47D cell line |
Bioorg Med Chem Lett 16: 2729-33 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.020
BindingDB Entry DOI: 10.7270/Q24Q7TK9 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM209932
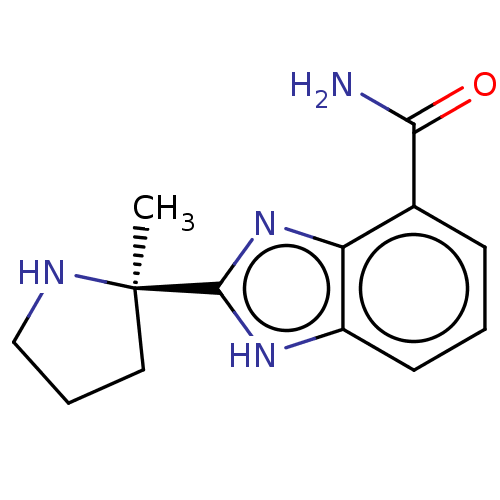
(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data