

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | High affinity nerve growth factor receptor | ||
| Ligand | BDBM127865 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | TrkA ELISA Assay | ||
| IC50 | 1.90±n/a nM | ||
| Citation |  Allen, S; Andrews, SW; Condroski, KR; Haas, J; Huang, L; Jiang, Y; Kercher, T; Seo, J Substituted pyrazolo[1,5-a]pyrimidine compounds as Trk kinase inhibitors US Patent US9782415 Publication Date 10/10/2017 Allen, S; Andrews, SW; Condroski, KR; Haas, J; Huang, L; Jiang, Y; Kercher, T; Seo, J Substituted pyrazolo[1,5-a]pyrimidine compounds as Trk kinase inhibitors US Patent US9782415 Publication Date 10/10/2017 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| High affinity nerve growth factor receptor | |||
| Name: | High affinity nerve growth factor receptor | ||
| Synonyms: | 2.7.10.1 | MTC | NTRK1 | NTRK1/NTRK2 | NTRK1_HUMAN | Nerve growth factor receptor Trk-A | Neurotrophic tyrosine kinase receptor type 1 | Neurotrophic tyrosine kinase receptor type 1 (TrkA) | Synonyms=MTC | TRK | TRK1-transforming tyrosine kinase protein | TRKA | TRKA GN | TRKA GN | Trk-A | Tropomyosin alpha-3 chain/High affinity nerve growth factor receptor | Tropomyosin-related kinase A | Tropomyosin-related kinase A (TrkA) | Tyrosine kinase receptor | Tyrosine kinase receptor (Trk) | Tyrosine kinase receptor A | Tyrosine kinase receptor A (Trk A) | Tyrosine kinase receptor A (Trk-A) | Tyrosine kinase receptor A (TrkA) | gp140trk | p140-TrkA | ||
| Type: | n/a | ||
| Mol. Mass.: | 87498.18 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P04629 | ||
| Residue: | 796 | ||
| Sequence: |
| ||
| BDBM127865 | |||
| n/a | |||
| Name | BDBM127865 | ||
| Synonyms: | US10251889, Example 238 | US10758542, Example 238 | US8791123, 238 | US9782415, Example 238 | US9796724, Example 238 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C23H27FN6O3 | ||
| Mol. Mass. | 454.4973 | ||
| SMILES | Cn1cc(F)cc([C@H]2CCCN2c2ccn3ncc(C(=O)NC4CC[C@H](O)CC4)c3n2)c1=O |r,wD:7.6,25.26,(-7.44,5.2,;-5.95,4.81,;-4.86,5.9,;-3.37,5.5,;-2.28,6.59,;-2.97,4.01,;-4.06,2.92,;-3.66,1.43,;-4.57,.19,;-3.66,-1.06,;-2.2,-.58,;-2.2,.96,;-.87,1.73,;-.87,3.27,;.47,4.04,;1.8,3.27,;3.27,3.74,;4.17,2.5,;3.27,1.25,;3.66,-.24,;2.58,-1.33,;5.15,-.64,;5.55,-2.12,;7.04,-2.52,;7.44,-4.01,;6.35,-5.1,;6.75,-6.59,;4.86,-4.7,;4.46,-3.21,;1.8,1.73,;.47,.96,;-5.55,3.32,;-6.64,2.23,)| | ||
| Structure | 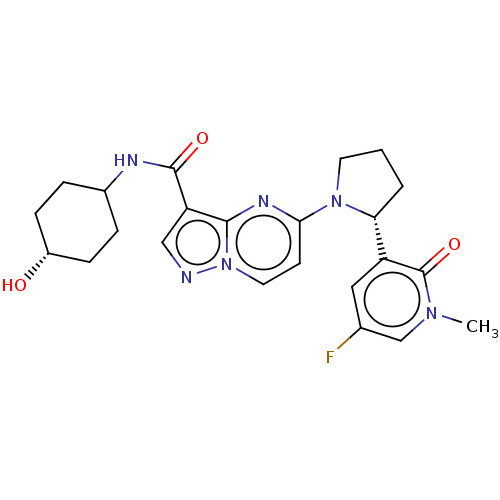
| ||