| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM10732 |
|---|
| Substrate/Competitor | BDBM10759 |
|---|
| Meas. Tech. | Cholinesterase Inhibition Assay |
|---|
| pH | 7.4±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| IC50 | 300±n/a nM |
|---|
| Citation |  Sterling, J; Herzig, Y; Goren, T; Finkelstein, N; Lerner, D; Goldenberg, W; Miskolczi, I; Molnar, S; Rantal, F; Tamas, T; Toth, G; Zagyva, A; Zekany, A; Finberg, J; Lavian, G; Gross, A; Friedman, R; Razin, M; Huang, W; Krais, B; Chorev, M; Youdim, MB; Weinstock, M Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem45:5260-79 (2002) [PubMed] Article Sterling, J; Herzig, Y; Goren, T; Finkelstein, N; Lerner, D; Goldenberg, W; Miskolczi, I; Molnar, S; Rantal, F; Tamas, T; Toth, G; Zagyva, A; Zekany, A; Finberg, J; Lavian, G; Gross, A; Friedman, R; Razin, M; Huang, W; Krais, B; Chorev, M; Youdim, MB; Weinstock, M Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem45:5260-79 (2002) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM10732 |
|---|
| BDBM10759 |
|---|
| Name | BDBM10732 |
|---|
| Synonyms: | 3-amino-2,3-dihydro-1H-inden-5-yl N-(4-methoxyphenyl)-N-methylcarbamate | Aminoindan deriv. 7f |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H20N2O3 |
|---|
| Mol. Mass. | 312.363 |
|---|
| SMILES | COc1ccc(cc1)N(C)C(=O)Oc1ccc2CCC(N)c2c1 |
|---|
| Structure | 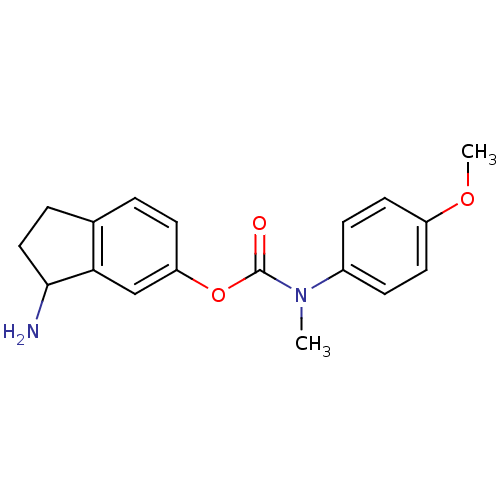
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sterling, J; Herzig, Y; Goren, T; Finkelstein, N; Lerner, D; Goldenberg, W; Miskolczi, I; Molnar, S; Rantal, F; Tamas, T; Toth, G; Zagyva, A; Zekany, A; Finberg, J; Lavian, G; Gross, A; Friedman, R; Razin, M; Huang, W; Krais, B; Chorev, M; Youdim, MB; Weinstock, M Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem45:5260-79 (2002) [PubMed] Article
Sterling, J; Herzig, Y; Goren, T; Finkelstein, N; Lerner, D; Goldenberg, W; Miskolczi, I; Molnar, S; Rantal, F; Tamas, T; Toth, G; Zagyva, A; Zekany, A; Finberg, J; Lavian, G; Gross, A; Friedman, R; Razin, M; Huang, W; Krais, B; Chorev, M; Youdim, MB; Weinstock, M Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem45:5260-79 (2002) [PubMed] Article