| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50240469 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1668231 (CHEMBL4018119) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Sakurada, I; Endo, T; Hikita, K; Hirabayashi, T; Hosaka, Y; Kato, Y; Maeda, Y; Matsumoto, S; Mizuno, T; Nagasue, H; Nishimura, T; Shimada, S; Shinozaki, M; Taguchi, K; Takeuchi, K; Yokoyama, T; Hruza, A; Reichert, P; Zhang, T; Wood, HB; Nakao, K; Furusako, S Discovery of novel aminobenzisoxazole derivatives as orally available factor IXa inhibitors. Bioorg Med Chem Lett27:2622-2628 (2017) [PubMed] Article Sakurada, I; Endo, T; Hikita, K; Hirabayashi, T; Hosaka, Y; Kato, Y; Maeda, Y; Matsumoto, S; Mizuno, T; Nagasue, H; Nishimura, T; Shimada, S; Shinozaki, M; Taguchi, K; Takeuchi, K; Yokoyama, T; Hruza, A; Reichert, P; Zhang, T; Wood, HB; Nakao, K; Furusako, S Discovery of novel aminobenzisoxazole derivatives as orally available factor IXa inhibitors. Bioorg Med Chem Lett27:2622-2628 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50240469 |
|---|
| n/a |
|---|
| Name | BDBM50240469 |
|---|
| Synonyms: | CHEMBL4096341 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H19Cl2N7O2 |
|---|
| Mol. Mass. | 484.338 |
|---|
| SMILES | [H][C@@]12CC[C@]([H])([C@H]1NC(=O)c1ccc(cc1Cl)-n1cnc(C)n1)N(C2)c1ncc(Cl)c2cnoc12 |r,TLB:7:6:23.24:2.3,THB:25:23:6:2.3| |
|---|
| Structure | 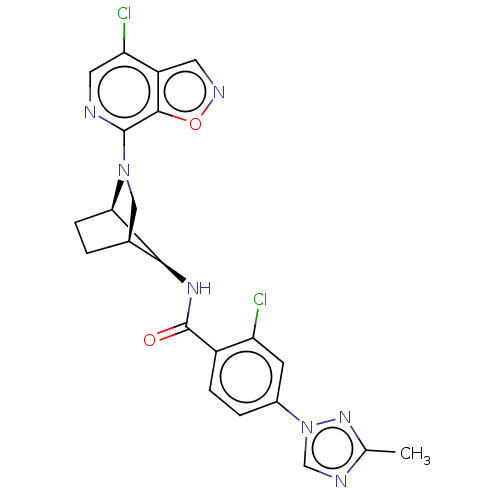
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sakurada, I; Endo, T; Hikita, K; Hirabayashi, T; Hosaka, Y; Kato, Y; Maeda, Y; Matsumoto, S; Mizuno, T; Nagasue, H; Nishimura, T; Shimada, S; Shinozaki, M; Taguchi, K; Takeuchi, K; Yokoyama, T; Hruza, A; Reichert, P; Zhang, T; Wood, HB; Nakao, K; Furusako, S Discovery of novel aminobenzisoxazole derivatives as orally available factor IXa inhibitors. Bioorg Med Chem Lett27:2622-2628 (2017) [PubMed] Article
Sakurada, I; Endo, T; Hikita, K; Hirabayashi, T; Hosaka, Y; Kato, Y; Maeda, Y; Matsumoto, S; Mizuno, T; Nagasue, H; Nishimura, T; Shimada, S; Shinozaki, M; Taguchi, K; Takeuchi, K; Yokoyama, T; Hruza, A; Reichert, P; Zhang, T; Wood, HB; Nakao, K; Furusako, S Discovery of novel aminobenzisoxazole derivatives as orally available factor IXa inhibitors. Bioorg Med Chem Lett27:2622-2628 (2017) [PubMed] Article