| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase |
|---|
| Ligand | BDBM50484031 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_755374 (CHEMBL1804530) |
|---|
| Ki | 12±n/a nM |
|---|
| Citation |  Su, DS; Lim, JJ; Tinney, E; Tucker, TJ; Saggar, S; Sisko, JT; Wan, BL; Young, MB; Anderson, KD; Rudd, D; Munshi, V; Bahnck, C; Felock, PJ; Lu, M; Lai, MT; Touch, S; Moyer, G; Distefano, DJ; Flynn, JA; Liang, Y; Sanchez, R; Perlow-Poehnelt, R; Miller, M; Vacca, JP; Williams, TM; Anthony, NJ Biaryl ethers as potent allosteric inhibitors of reverse transcriptase and its key mutant viruses: aryl substituted pyrazole as a surrogate for the pyrazolopyridine motif. Bioorg Med Chem Lett20:4328-32 (2010) [PubMed] Article Su, DS; Lim, JJ; Tinney, E; Tucker, TJ; Saggar, S; Sisko, JT; Wan, BL; Young, MB; Anderson, KD; Rudd, D; Munshi, V; Bahnck, C; Felock, PJ; Lu, M; Lai, MT; Touch, S; Moyer, G; Distefano, DJ; Flynn, JA; Liang, Y; Sanchez, R; Perlow-Poehnelt, R; Miller, M; Vacca, JP; Williams, TM; Anthony, NJ Biaryl ethers as potent allosteric inhibitors of reverse transcriptase and its key mutant viruses: aryl substituted pyrazole as a surrogate for the pyrazolopyridine motif. Bioorg Med Chem Lett20:4328-32 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase |
|---|
| Name: | Reverse transcriptase |
|---|
| Synonyms: | n/a |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 29598.37 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | Q9WKE8 |
|---|
| Residue: | 254 |
|---|
| Sequence: | PISPITVPVKLKPGMDGPKVKQWPLTEEKIKALTEICTEMEKEGKIEKIGPENPYNTPVF
AIKKKDSTKWRKVVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLD
KDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVIY
QYMDDLYVGSDLEIEQHRAKIEELRQHLLRWGFTTPDKKHQKEPPFLWMGYELHPDKWTV
QPIVLPEKDSWTVN
|
|
|
|---|
| BDBM50484031 |
|---|
| n/a |
|---|
| Name | BDBM50484031 |
|---|
| Synonyms: | CHEMBL1801255 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H14Cl2N4O2 |
|---|
| Mol. Mass. | 461.3 |
|---|
| SMILES | Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccc(cc3)C#N)ccc2Cl)cc(c1)C#N |
|---|
| Structure | 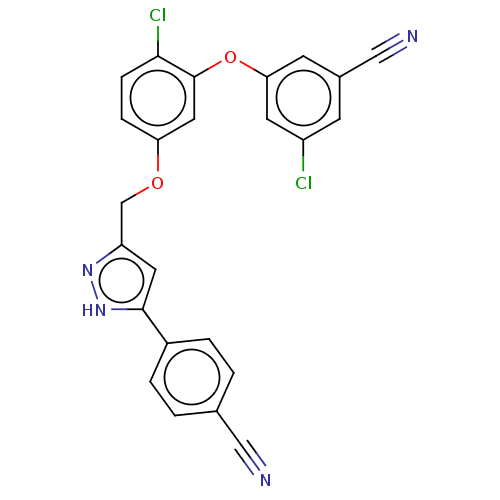
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Su, DS; Lim, JJ; Tinney, E; Tucker, TJ; Saggar, S; Sisko, JT; Wan, BL; Young, MB; Anderson, KD; Rudd, D; Munshi, V; Bahnck, C; Felock, PJ; Lu, M; Lai, MT; Touch, S; Moyer, G; Distefano, DJ; Flynn, JA; Liang, Y; Sanchez, R; Perlow-Poehnelt, R; Miller, M; Vacca, JP; Williams, TM; Anthony, NJ Biaryl ethers as potent allosteric inhibitors of reverse transcriptase and its key mutant viruses: aryl substituted pyrazole as a surrogate for the pyrazolopyridine motif. Bioorg Med Chem Lett20:4328-32 (2010) [PubMed] Article
Su, DS; Lim, JJ; Tinney, E; Tucker, TJ; Saggar, S; Sisko, JT; Wan, BL; Young, MB; Anderson, KD; Rudd, D; Munshi, V; Bahnck, C; Felock, PJ; Lu, M; Lai, MT; Touch, S; Moyer, G; Distefano, DJ; Flynn, JA; Liang, Y; Sanchez, R; Perlow-Poehnelt, R; Miller, M; Vacca, JP; Williams, TM; Anthony, NJ Biaryl ethers as potent allosteric inhibitors of reverse transcriptase and its key mutant viruses: aryl substituted pyrazole as a surrogate for the pyrazolopyridine motif. Bioorg Med Chem Lett20:4328-32 (2010) [PubMed] Article