

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50184069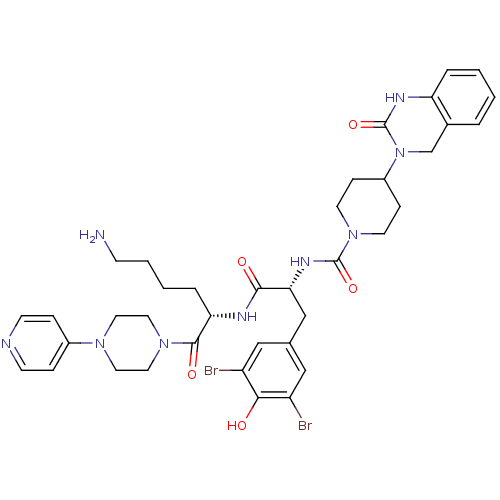 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry Merck & Co. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from human cloned CLR/RAMP1 receptor expressed in E10 cells | Bioorg Med Chem Lett 16: 2595-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.051 BindingDB Entry DOI: 10.7270/Q2HT2NX8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156449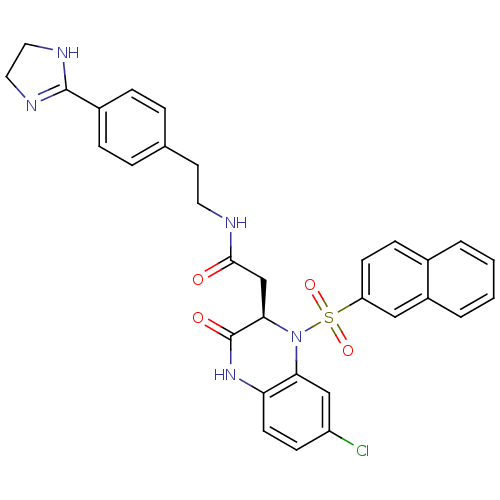 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156446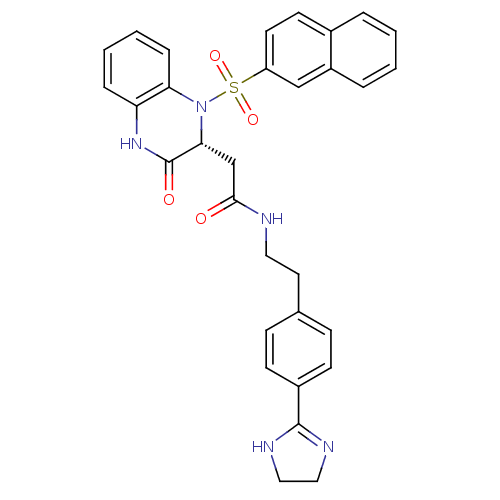 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to rhesus monkey Bradykinin receptor B1 | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156451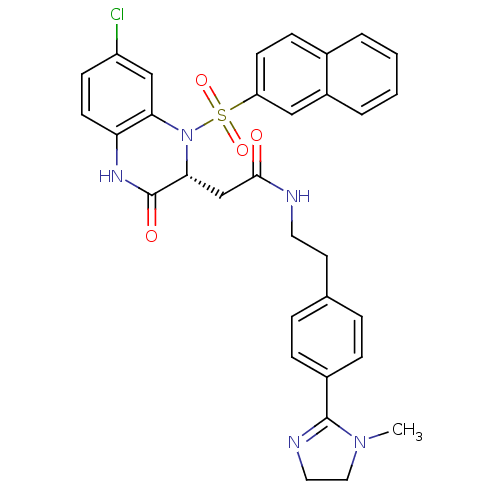 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156446 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo rec... | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455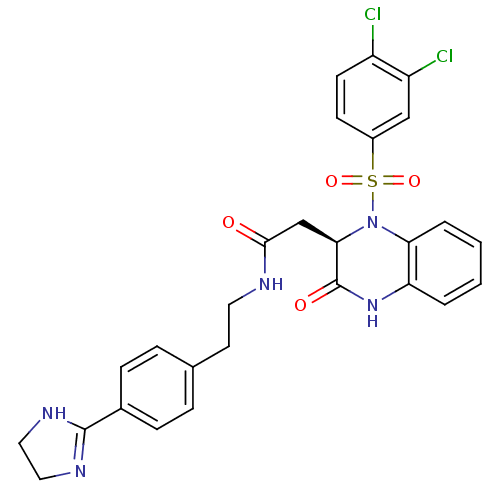 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Oryctolagus cuniculus) | BDBM50156446 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to rabbit Bradykinin receptor B1 | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156448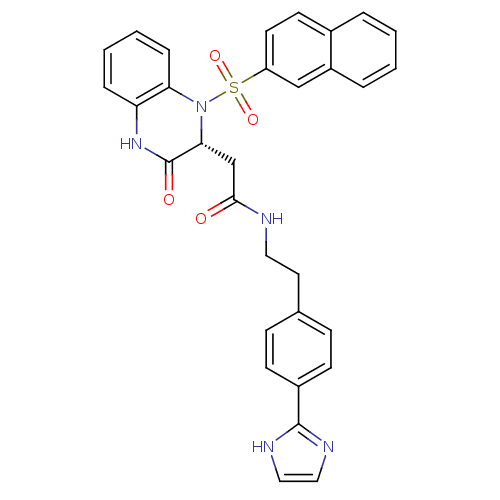 (CHEMBL185811 | N-{2-[4-(1H-Imidazol-2-yl)-phenyl]-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156450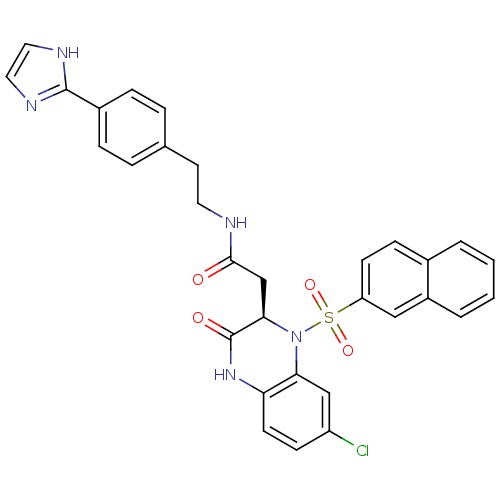 (2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486093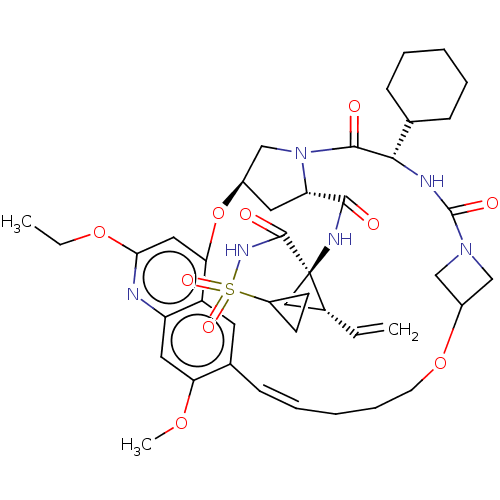 (CHEMBL2203889) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471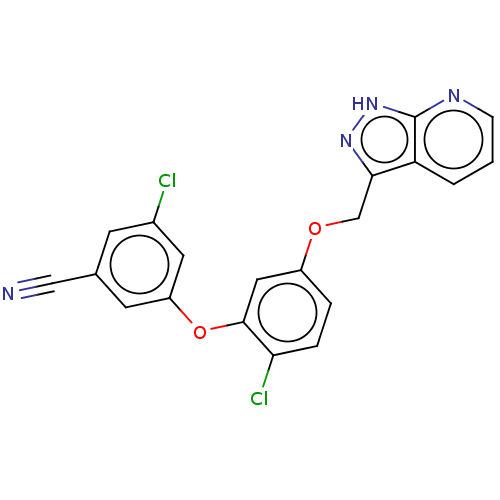 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50243173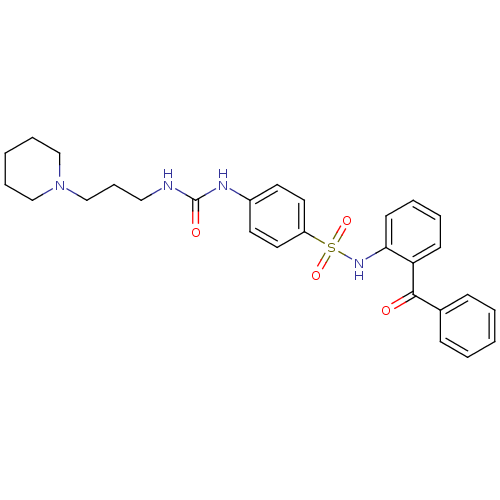 (CHEMBL487445 | N-(2-Benzoyl-phenyl)-4-[3-(3-piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradykinin B1 receptor | J Med Chem 51: 3946-52 (2008) Article DOI: 10.1021/jm800199h BindingDB Entry DOI: 10.7270/Q2JH3N33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029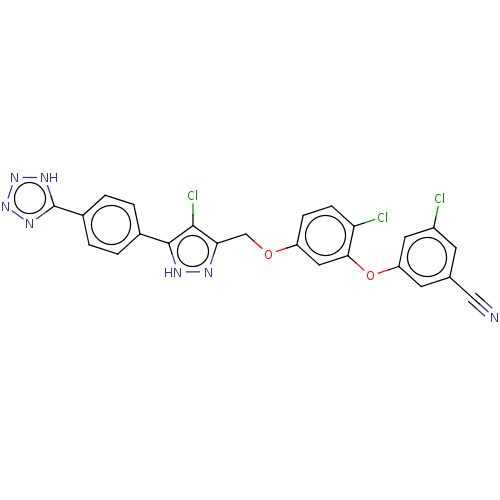 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484039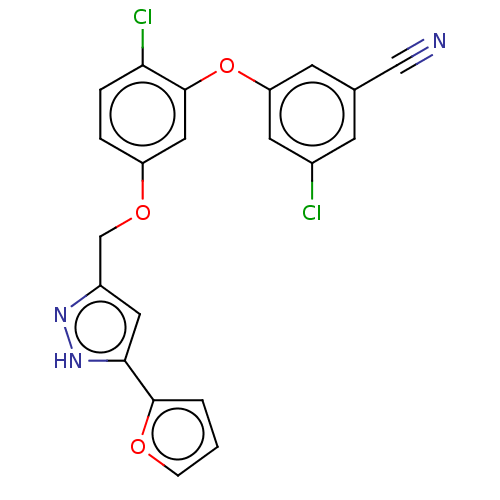 (CHEMBL1800087) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371333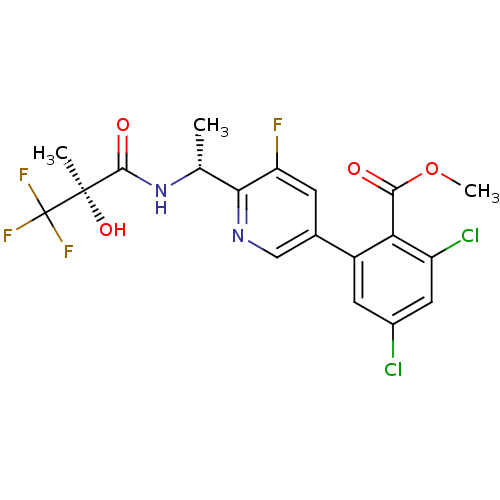 (CHEMBL256671) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50210360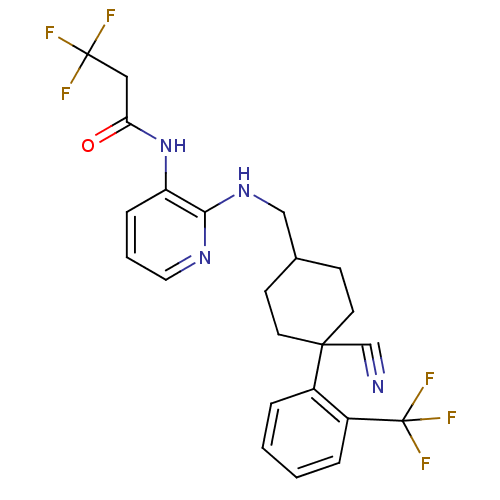 (CHEMBL234096 | N-(2-((4-cyano-4-(2-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity at human bradykinin B1 receptor | Bioorg Med Chem Lett 17: 3006-9 (2007) Article DOI: 10.1016/j.bmcl.2007.03.059 BindingDB Entry DOI: 10.7270/Q29C6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486108 (CHEMBL2203884) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484635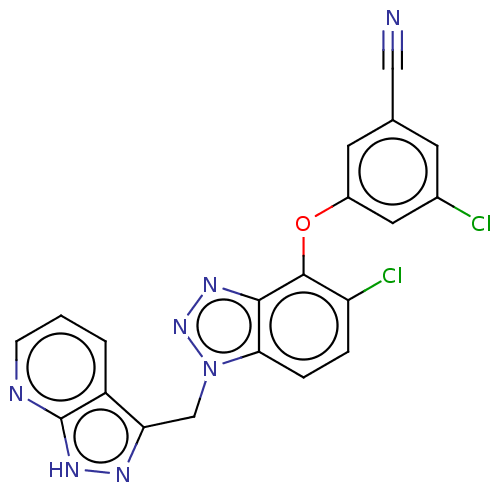 (CHEMBL1939500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371320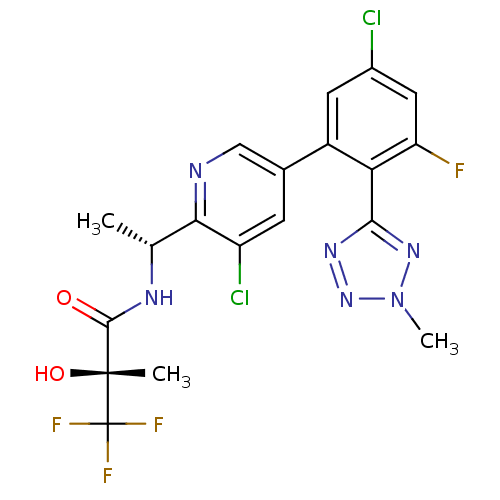 (CHEMBL271283) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484496 (CHEMBL1928648) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labs. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 21: 7344-50 (2011) Article DOI: 10.1016/j.bmcl.2011.10.027 BindingDB Entry DOI: 10.7270/Q25Q4ZZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045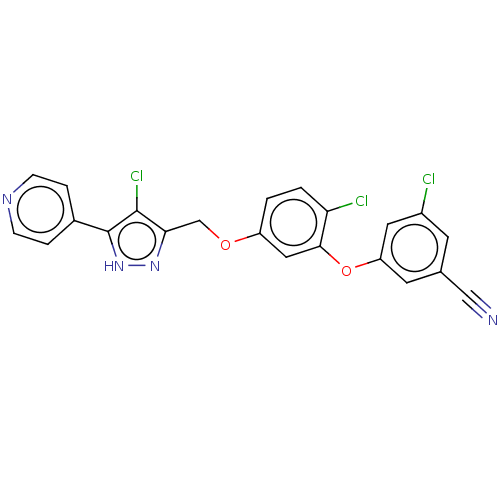 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Canis familiaris) | BDBM50156446 (CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the [35S]- radiolabelled compound to dog Bradykinin receptor B1 | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484632 (Mk-6186 | Mk6186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371332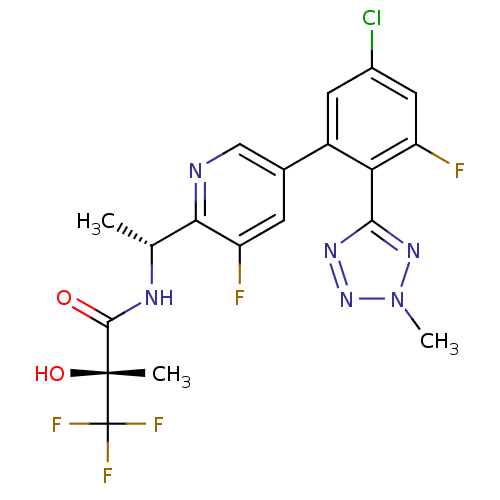 (CHEMBL258324) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484030 (CHEMBL1801256) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50210361 (CHEMBL234097 | N-(2-((4-cyano-4-(2-(trifluoromethy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity at human bradykinin B1 receptor | Bioorg Med Chem Lett 17: 3006-9 (2007) Article DOI: 10.1016/j.bmcl.2007.03.059 BindingDB Entry DOI: 10.7270/Q29C6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371321 (CHEMBL258326) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50229295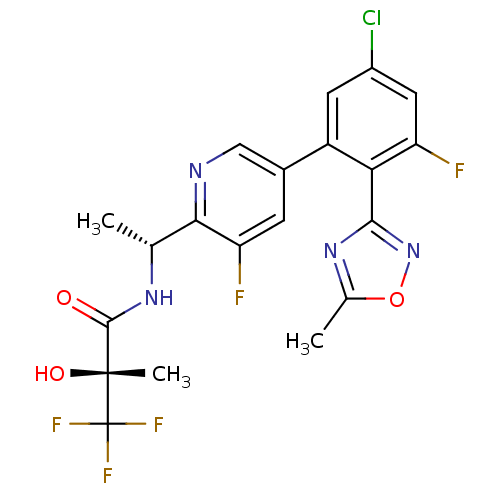 ((R)-N-((R)-1-(5-(5-chloro-3-fluoro-2-(5-methyl-1,2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486101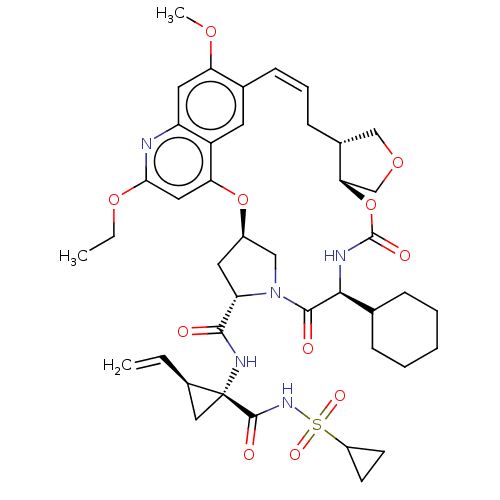 (CHEMBL2203879) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484029 (CHEMBL1801258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484031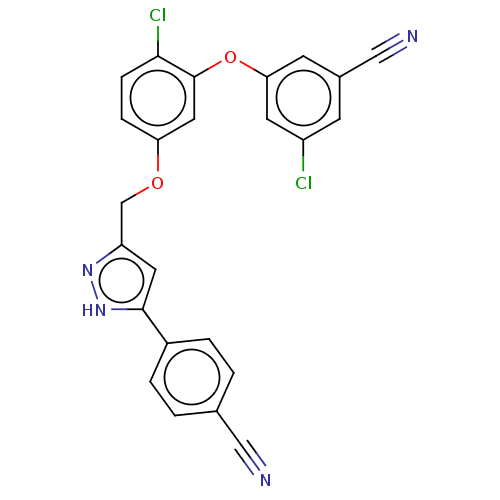 (CHEMBL1801255) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484045 (CHEMBL1801257) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486111 (CHEMBL2203888) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484040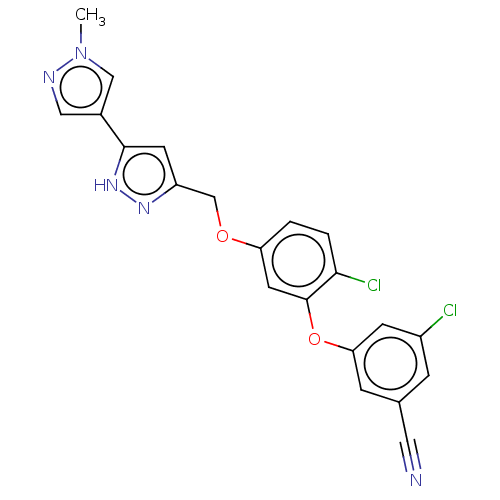 (CHEMBL1801228) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50243301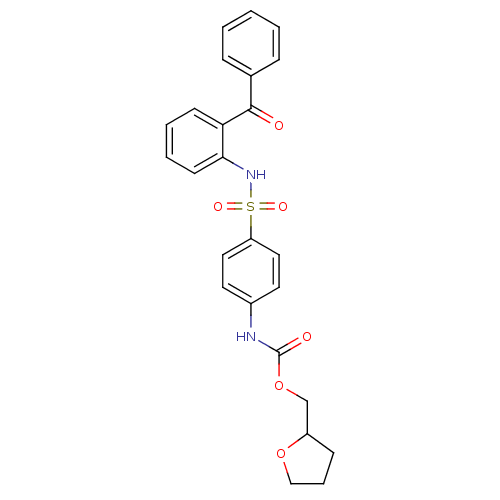 (CHEMBL454085 | Tetrahydrofuran-2-ylmethyl 4-{[(2-b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradykinin B1 receptor | J Med Chem 51: 3946-52 (2008) Article DOI: 10.1021/jm800199h BindingDB Entry DOI: 10.7270/Q2JH3N33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484032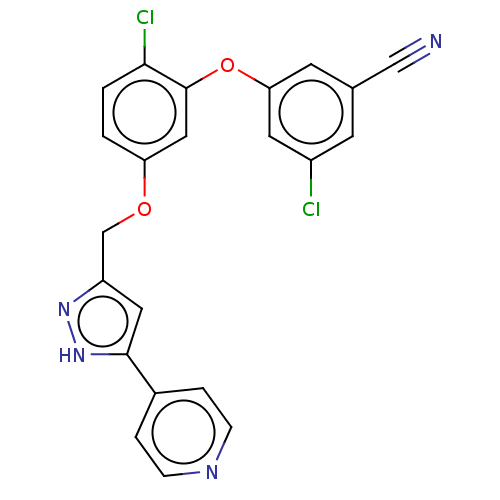 (CHEMBL1801231) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50371322 (CHEMBL257729) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human bradikinin B1 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 716-20 (2008) Article DOI: 10.1016/j.bmcl.2007.11.050 BindingDB Entry DOI: 10.7270/Q2GM8849 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486103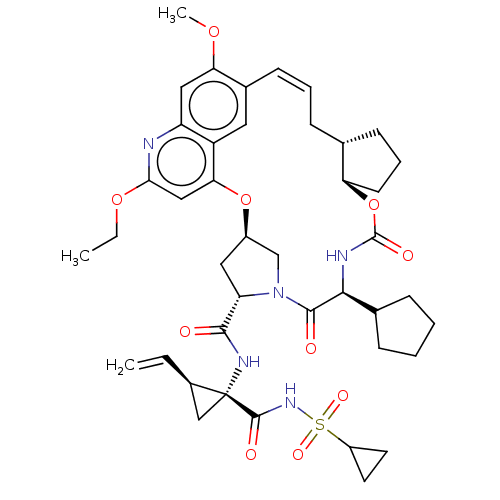 (CHEMBL2203874) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484022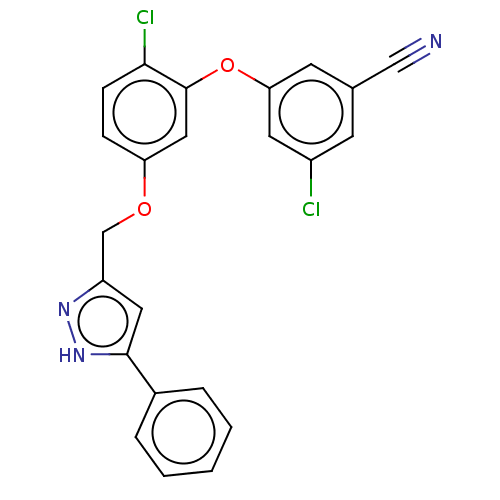 (CHEMBL1801223) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484629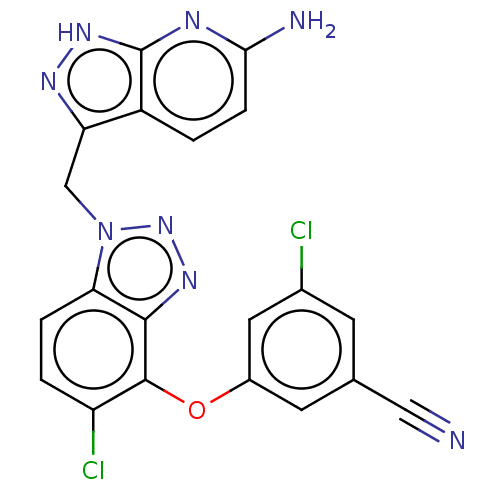 (CHEMBL1939503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156452 (2-[(R)-1-(3,4-Dichloro-benzenesulfonyl)-3-oxo-1,2,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay | Bioorg Med Chem Lett 14: 6045-8 (2004) Article DOI: 10.1016/j.bmcl.2004.09.074 BindingDB Entry DOI: 10.7270/Q2V1248Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484023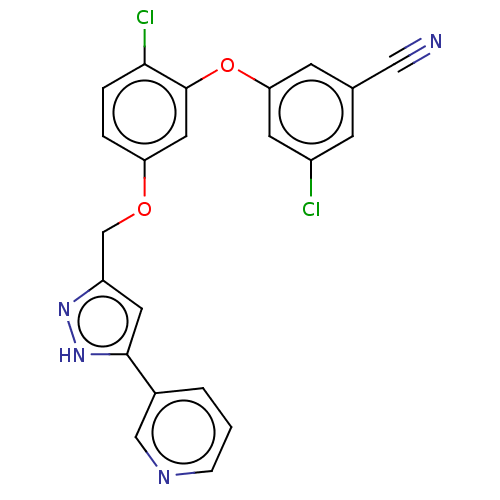 (CHEMBL1801230) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase by SPA assay | Bioorg Med Chem Lett 20: 4328-32 (2010) Article DOI: 10.1016/j.bmcl.2010.06.083 BindingDB Entry DOI: 10.7270/Q2TH8QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 757 total ) | Next | Last >> |