| Reaction Details |
|---|
 | Report a problem with these data |
| Target | RNA-directed RNA polymerase |
|---|
| Ligand | BDBM50274118 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1892471 (CHEMBL4394392) |
|---|
| IC50 | 140±n/a nM |
|---|
| Citation |  Wang, G; Dyatkina, N; Prhavc, M; Williams, C; Serebryany, V; Hu, Y; Huang, Y; Wan, J; Wu, X; Deval, J; Fung, A; Jin, Z; Tan, H; Shaw, K; Kang, H; Zhang, Q; Tam, Y; Stoycheva, A; Jekle, A; Smith, DB; Beigelman, L Synthesis and Anti-HCV Activities of 4'-Fluoro-2'-Substituted Uridine Triphosphates and Nucleotide Prodrugs: Discovery of 4'-Fluoro-2'- C-methyluridine 5'-Phosphoramidate Prodrug (AL-335) for the Treatment of Hepatitis C Infection. J Med Chem62:4555-4570 (2019) [PubMed] Article Wang, G; Dyatkina, N; Prhavc, M; Williams, C; Serebryany, V; Hu, Y; Huang, Y; Wan, J; Wu, X; Deval, J; Fung, A; Jin, Z; Tan, H; Shaw, K; Kang, H; Zhang, Q; Tam, Y; Stoycheva, A; Jekle, A; Smith, DB; Beigelman, L Synthesis and Anti-HCV Activities of 4'-Fluoro-2'-Substituted Uridine Triphosphates and Nucleotide Prodrugs: Discovery of 4'-Fluoro-2'- C-methyluridine 5'-Phosphoramidate Prodrug (AL-335) for the Treatment of Hepatitis C Infection. J Med Chem62:4555-4570 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| RNA-directed RNA polymerase |
|---|
| Name: | RNA-directed RNA polymerase |
|---|
| Synonyms: | Hepatitis C virus NS5B RNA-dependent RNA polymerase | NS5B protein |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 25173.95 |
|---|
| Organism: | Hepatitis C virus |
|---|
| Description: | Q8JXU8 |
|---|
| Residue: | 229 |
|---|
| Sequence: | RTEEAIYQCCDLDPQARVAIRSLTERLYVGGPLTNSRGENCGYRRRASGVLTTSCGNTLT
CYIKAQAACRAAGRQDCTMLVCGDDLVVICESAGVQEDAASLRAFTEAMTRYSAPPGDPP
QPEYDLELITSCSSNVSVAHDGAGKRVYYLTRDPTTPLARAAWETARHTPVNSWLGNIIM
FAPTLWVRMIMLTHFFSVLIARDQLEQALDCEIYGACYSIEPLLPPIIQ
|
|
|
|---|
| BDBM50274118 |
|---|
| n/a |
|---|
| Name | BDBM50274118 |
|---|
| Synonyms: | CHEMBL4127030 | US10793591, Compound 7a |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H16FN2O15P3 |
|---|
| Mol. Mass. | 516.1581 |
|---|
| SMILES | C[C@@]1(O)[C@H](O)[C@@](F)(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(=O)[nH]c1=O |r| |
|---|
| Structure | 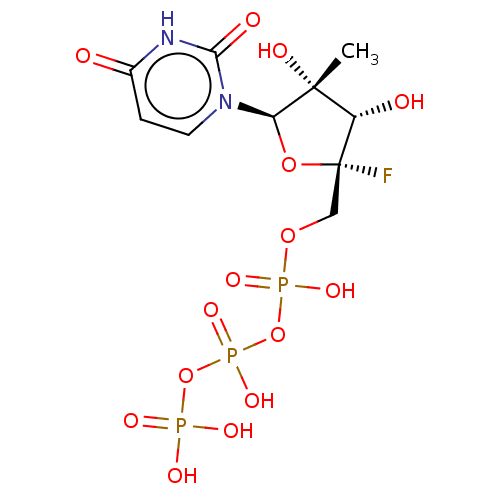
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wang, G; Dyatkina, N; Prhavc, M; Williams, C; Serebryany, V; Hu, Y; Huang, Y; Wan, J; Wu, X; Deval, J; Fung, A; Jin, Z; Tan, H; Shaw, K; Kang, H; Zhang, Q; Tam, Y; Stoycheva, A; Jekle, A; Smith, DB; Beigelman, L Synthesis and Anti-HCV Activities of 4'-Fluoro-2'-Substituted Uridine Triphosphates and Nucleotide Prodrugs: Discovery of 4'-Fluoro-2'- C-methyluridine 5'-Phosphoramidate Prodrug (AL-335) for the Treatment of Hepatitis C Infection. J Med Chem62:4555-4570 (2019) [PubMed] Article
Wang, G; Dyatkina, N; Prhavc, M; Williams, C; Serebryany, V; Hu, Y; Huang, Y; Wan, J; Wu, X; Deval, J; Fung, A; Jin, Z; Tan, H; Shaw, K; Kang, H; Zhang, Q; Tam, Y; Stoycheva, A; Jekle, A; Smith, DB; Beigelman, L Synthesis and Anti-HCV Activities of 4'-Fluoro-2'-Substituted Uridine Triphosphates and Nucleotide Prodrugs: Discovery of 4'-Fluoro-2'- C-methyluridine 5'-Phosphoramidate Prodrug (AL-335) for the Treatment of Hepatitis C Infection. J Med Chem62:4555-4570 (2019) [PubMed] Article