| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50262487 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_514589 (CHEMBL979967) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Sweeney, ZK; Dunn, JP; Li, Y; Heilek, G; Dunten, P; Elworthy, TR; Han, X; Harris, SF; Hirschfeld, DR; Hogg, JH; Huber, W; Kaiser, AC; Kertesz, DJ; Kim, W; Mirzadegan, T; Roepel, MG; Saito, YD; Silva, TM; Swallow, S; Tracy, JL; Villasenor, A; Vora, H; Zhou, AS; Klumpp, K Discovery and optimization of pyridazinone non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett18:4352-4 (2008) [PubMed] Article Sweeney, ZK; Dunn, JP; Li, Y; Heilek, G; Dunten, P; Elworthy, TR; Han, X; Harris, SF; Hirschfeld, DR; Hogg, JH; Huber, W; Kaiser, AC; Kertesz, DJ; Kim, W; Mirzadegan, T; Roepel, MG; Saito, YD; Silva, TM; Swallow, S; Tracy, JL; Villasenor, A; Vora, H; Zhou, AS; Klumpp, K Discovery and optimization of pyridazinone non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett18:4352-4 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50262487 |
|---|
| n/a |
|---|
| Name | BDBM50262487 |
|---|
| Synonyms: | 3-(6-chloro-2-fluoro-3-((5-methyl-6-oxo-1,6-dihydropyridazin-3-yl)methyl)phenoxy)-5-fluorobenzonitrile | CHEMBL477145 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H12ClF2N3O2 |
|---|
| Mol. Mass. | 387.767 |
|---|
| SMILES | Cc1cc(Cc2ccc(Cl)c(Oc3cc(F)cc(c3)C#N)c2F)n[nH]c1=O |
|---|
| Structure | 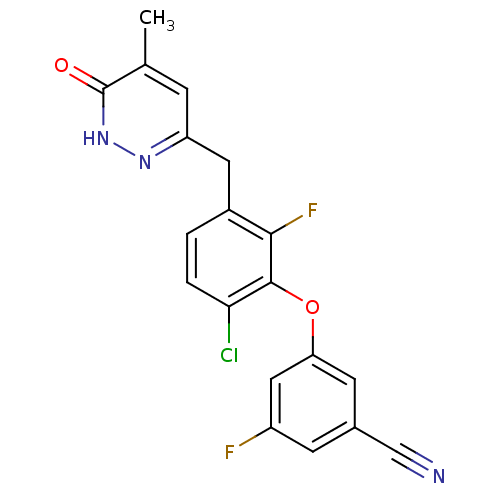
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sweeney, ZK; Dunn, JP; Li, Y; Heilek, G; Dunten, P; Elworthy, TR; Han, X; Harris, SF; Hirschfeld, DR; Hogg, JH; Huber, W; Kaiser, AC; Kertesz, DJ; Kim, W; Mirzadegan, T; Roepel, MG; Saito, YD; Silva, TM; Swallow, S; Tracy, JL; Villasenor, A; Vora, H; Zhou, AS; Klumpp, K Discovery and optimization of pyridazinone non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett18:4352-4 (2008) [PubMed] Article
Sweeney, ZK; Dunn, JP; Li, Y; Heilek, G; Dunten, P; Elworthy, TR; Han, X; Harris, SF; Hirschfeld, DR; Hogg, JH; Huber, W; Kaiser, AC; Kertesz, DJ; Kim, W; Mirzadegan, T; Roepel, MG; Saito, YD; Silva, TM; Swallow, S; Tracy, JL; Villasenor, A; Vora, H; Zhou, AS; Klumpp, K Discovery and optimization of pyridazinone non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett18:4352-4 (2008) [PubMed] Article