 Found 1289 hits with Last Name = 'huber' and Initial = 'w'
Found 1289 hits with Last Name = 'huber' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035500
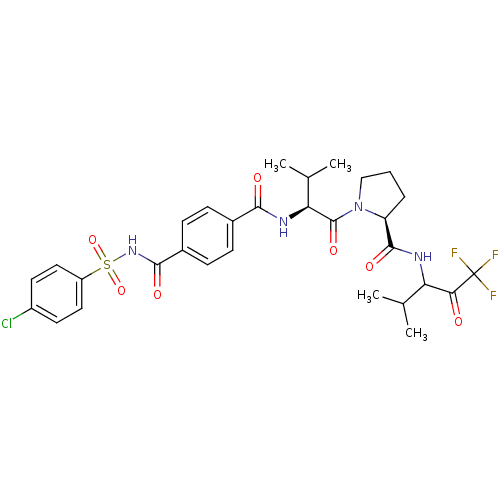
((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34ClF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035489
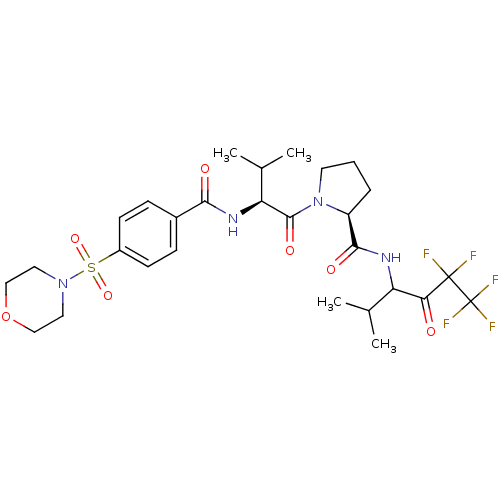
((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-sulfonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)S(=O)(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C28H37F5N4O7S/c1-16(2)21(23(38)27(29,30)28(31,32)33)34-25(40)20-6-5-11-37(20)26(41)22(17(3)4)35-24(39)18-7-9-19(10-8-18)45(42,43)36-12-14-44-15-13-36/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,34,40)(H,35,39)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035528
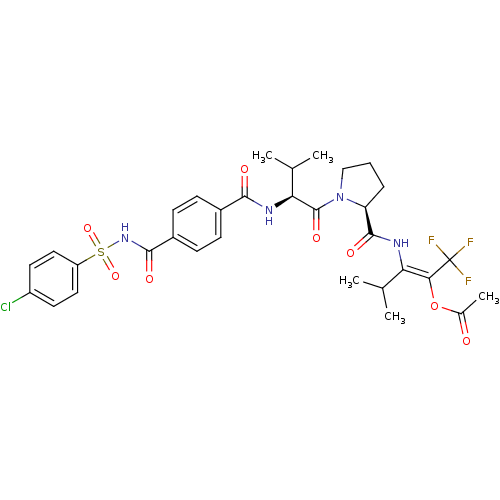
(Acetic acid (E)-2-[((S)-1-{(S)-2-[4-(4-chloro-benz...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)N\C(C(C)C)=C(\OC(C)=O)C(F)(F)F Show InChI InChI=1S/C32H36ClF3N4O8S/c1-17(2)25(27(32(34,35)36)48-19(5)41)37-30(44)24-7-6-16-40(24)31(45)26(18(3)4)38-28(42)20-8-10-21(11-9-20)29(43)39-49(46,47)23-14-12-22(33)13-15-23/h8-15,17-18,24,26H,6-7,16H2,1-5H3,(H,37,44)(H,38,42)(H,39,43)/b27-25+/t24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035500

((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34ClF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50324743
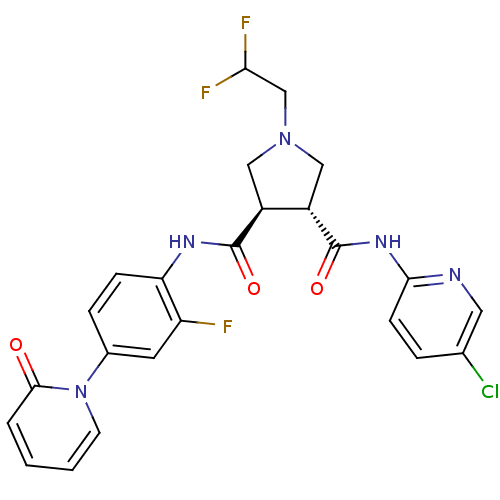
((3R,4R)-1-(2,2-DIFLUORO-ETHYL)-PYRROLIDINE-3,4-DIC...)Show SMILES FC(F)CN1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C24H21ClF3N5O3/c25-14-4-7-21(29-10-14)31-24(36)17-12-32(13-20(27)28)11-16(17)23(35)30-19-6-5-15(9-18(19)26)33-8-2-1-3-22(33)34/h1-10,16-17,20H,11-13H2,(H,30,35)(H,29,31,36)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037811

(CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)C(F)(F)C(=O)NCc1ccccc1 Show InChI InChI=1S/C38H39F2N3O6/c1-26(2)33(43-37(47)49-25-30-16-10-5-11-17-30)35(45)42-32(34(44)38(39,40)36(46)41-23-28-12-6-3-7-13-28)22-27-18-20-31(21-19-27)48-24-29-14-8-4-9-15-29/h3-21,26,32-33H,22-25H2,1-2H3,(H,41,46)(H,42,45)(H,43,47)/t32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the ability to inhibit HIV-protease. |
J Med Chem 37: 3684-92 (1994)
BindingDB Entry DOI: 10.7270/Q2ZW1JZT |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035488
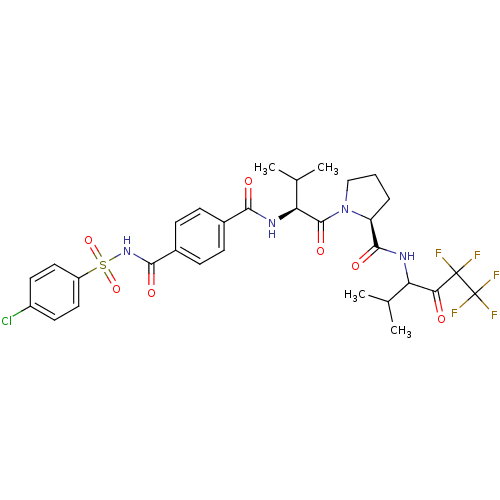
((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C31H34ClF5N4O7S/c1-16(2)23(25(42)30(33,34)31(35,36)37)38-28(45)22-6-5-15-41(22)29(46)24(17(3)4)39-26(43)18-7-9-19(10-8-18)27(44)40-49(47,48)21-13-11-20(32)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,38,45)(H,39,43)(H,40,44)/t22-,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 100 mg/kg |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324771
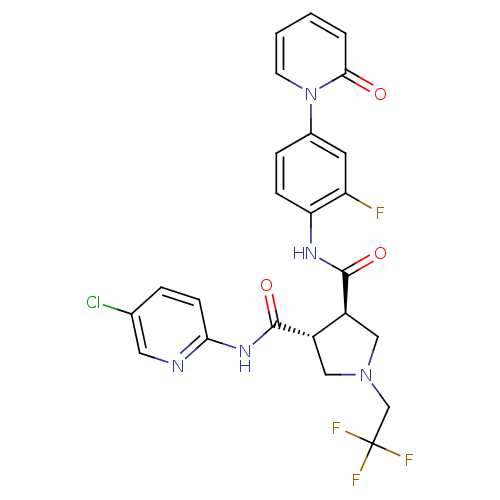
((3R,4R)-N3-(5-chloropyridin-2-yl)-N4-(2-fluoro-4-(...)Show SMILES Fc1cc(ccc1NC(=O)[C@H]1CN(CC(F)(F)F)C[C@@H]1C(=O)Nc1ccc(Cl)cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C24H20ClF4N5O3/c25-14-4-7-20(30-10-14)32-23(37)17-12-33(13-24(27,28)29)11-16(17)22(36)31-19-6-5-15(9-18(19)26)34-8-2-1-3-21(34)35/h1-10,16-17H,11-13H2,(H,31,36)(H,30,32,37)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324757
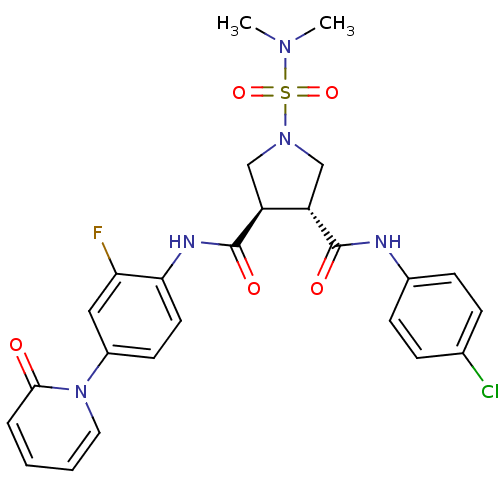
((3R,4R)-N3-(4-chlorophenyl)-1-(N,N-dimethylsulfamo...)Show SMILES CN(C)S(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H25ClFN5O5S/c1-30(2)38(36,37)31-14-19(24(34)28-17-8-6-16(26)7-9-17)20(15-31)25(35)29-22-11-10-18(13-21(22)27)32-12-4-3-5-23(32)33/h3-13,19-20H,14-15H2,1-2H3,(H,28,34)(H,29,35)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324755
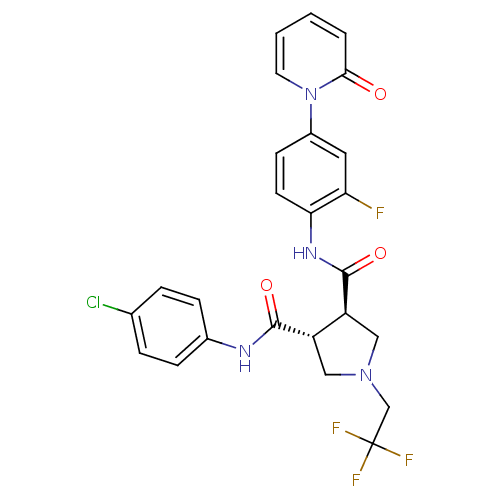
((3R,4R)-N-(4-CHLOROPHENYL)-N'-[2-FLUORO-4-(2-OXOPY...)Show SMILES Fc1cc(ccc1NC(=O)[C@H]1CN(CC(F)(F)F)C[C@@H]1C(=O)Nc1ccc(Cl)cc1)-n1ccccc1=O |r| Show InChI InChI=1S/C25H21ClF4N4O3/c26-15-4-6-16(7-5-15)31-23(36)18-12-33(14-25(28,29)30)13-19(18)24(37)32-21-9-8-17(11-20(21)27)34-10-2-1-3-22(34)35/h1-11,18-19H,12-14H2,(H,31,36)(H,32,37)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279944
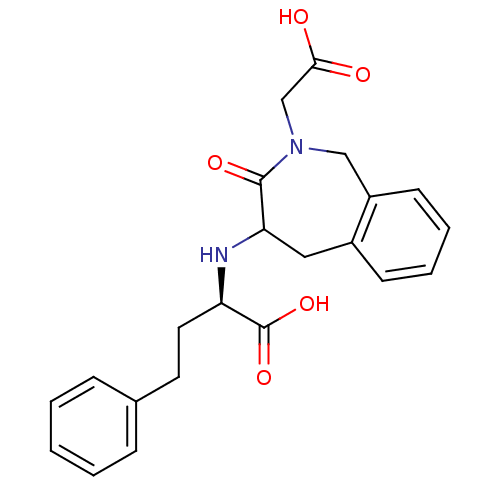
((R)-2-(2-Carboxymethyl-3-oxo-2,3,4,5-tetrahydro-1H...)Show SMILES OC(=O)CN1Cc2ccccc2CC(N[C@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-13-17-9-5-4-8-16(17)12-19(21(24)27)23-18(22(28)29)11-10-15-6-2-1-3-7-15/h1-9,18-19,23H,10-14H2,(H,25,26)(H,28,29)/t18-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound was tested angiotensin converting enzyme |
Bioorg Med Chem Lett 1: 309-312 (1991)
Article DOI: 10.1016/S0960-894X(01)80814-3
BindingDB Entry DOI: 10.7270/Q208657V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324758
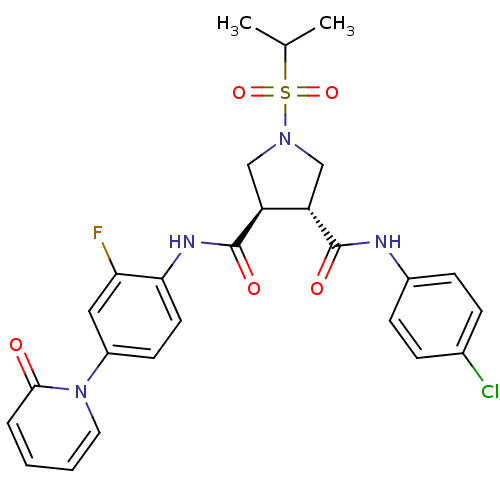
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES CC(C)S(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H26ClFN4O5S/c1-16(2)38(36,37)31-14-20(25(34)29-18-8-6-17(27)7-9-18)21(15-31)26(35)30-23-11-10-19(13-22(23)28)32-12-4-3-5-24(32)33/h3-13,16,20-21H,14-15H2,1-2H3,(H,29,34)(H,30,35)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324752
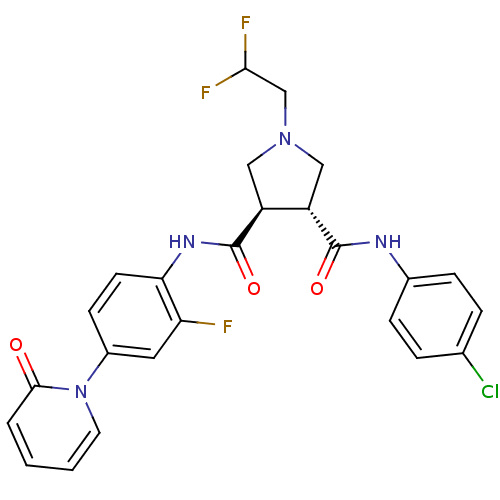
((3R,4R)-N3-(4-chlorophenyl)-1-(2,2-difluoroethyl)-...)Show SMILES FC(F)CN1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H22ClF3N4O3/c26-15-4-6-16(7-5-15)30-24(35)18-12-32(14-22(28)29)13-19(18)25(36)31-21-9-8-17(11-20(21)27)33-10-2-1-3-23(33)34/h1-11,18-19,22H,12-14H2,(H,30,35)(H,31,36)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324754
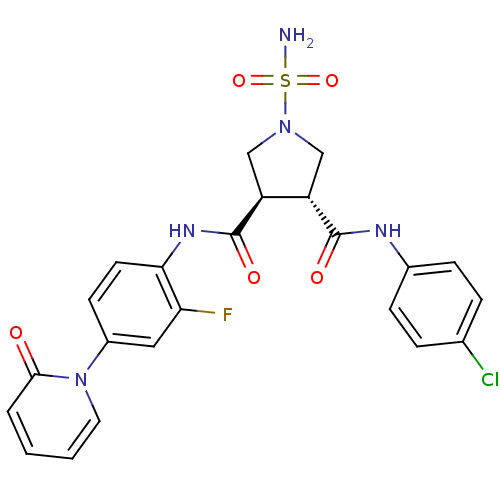
((3R,4R)-1-SULFAMOYL-PYRROLIDINE-3,4-DICARBOXYLIC A...)Show SMILES NS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H21ClFN5O5S/c24-14-4-6-15(7-5-14)27-22(32)17-12-29(36(26,34)35)13-18(17)23(33)28-20-9-8-16(11-19(20)25)30-10-2-1-3-21(30)31/h1-11,17-18H,12-13H2,(H,27,32)(H,28,33)(H2,26,34,35)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324767
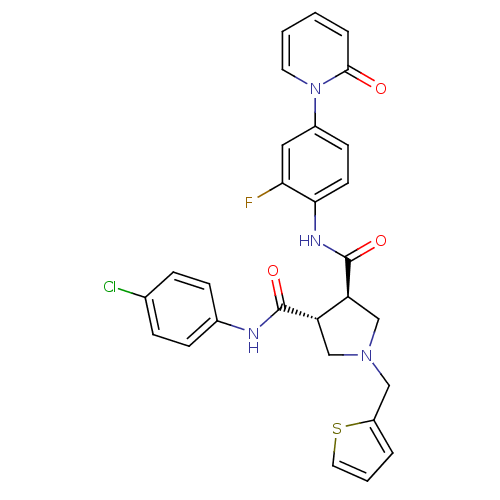
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES Fc1cc(ccc1NC(=O)[C@H]1CN(Cc2cccs2)C[C@@H]1C(=O)Nc1ccc(Cl)cc1)-n1ccccc1=O |r| Show InChI InChI=1S/C28H24ClFN4O3S/c29-18-6-8-19(9-7-18)31-27(36)22-16-33(15-21-4-3-13-38-21)17-23(22)28(37)32-25-11-10-20(14-24(25)30)34-12-2-1-5-26(34)35/h1-14,22-23H,15-17H2,(H,31,36)(H,32,37)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324749
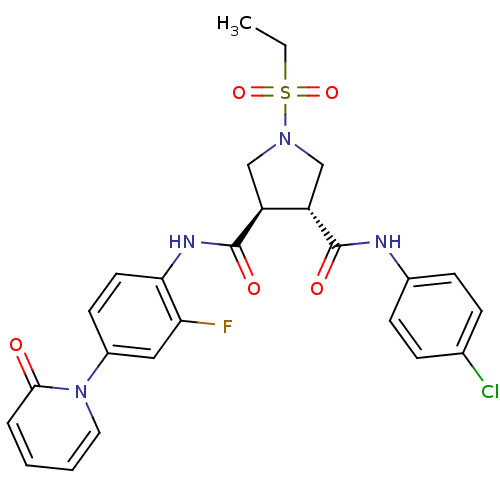
((3R,4R)-N3-(4-chlorophenyl)-1-(ethylsulfonyl)-N4-(...)Show SMILES CCS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H24ClFN4O5S/c1-2-37(35,36)30-14-19(24(33)28-17-8-6-16(26)7-9-17)20(15-30)25(34)29-22-11-10-18(13-21(22)27)31-12-4-3-5-23(31)32/h3-13,19-20H,2,14-15H2,1H3,(H,28,33)(H,29,34)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035498
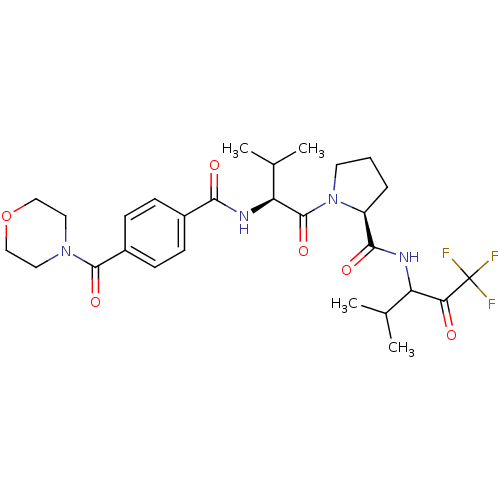
((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O6/c1-16(2)21(23(36)28(29,30)31)32-25(38)20-6-5-11-35(20)27(40)22(17(3)4)33-24(37)18-7-9-19(10-8-18)26(39)34-12-14-41-15-13-34/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,32,38)(H,33,37)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035498

((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C28H37F3N4O6/c1-16(2)21(23(36)28(29,30)31)32-25(38)20-6-5-11-35(20)27(40)22(17(3)4)33-24(37)18-7-9-19(10-8-18)26(39)34-12-14-41-15-13-34/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,32,38)(H,33,37)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324787
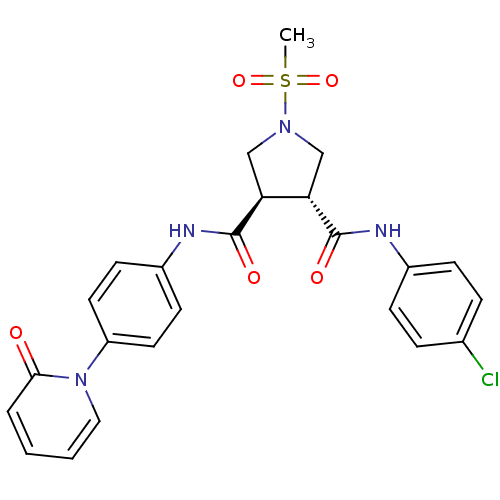
((3R,4R)-N3-(4-chlorophenyl)-1-(methylsulfonyl)-N4-...)Show SMILES CS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H23ClN4O5S/c1-35(33,34)28-14-20(23(31)26-17-7-5-16(25)6-8-17)21(15-28)24(32)27-18-9-11-19(12-10-18)29-13-3-2-4-22(29)30/h2-13,20-21H,14-15H2,1H3,(H,26,31)(H,27,32)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324744
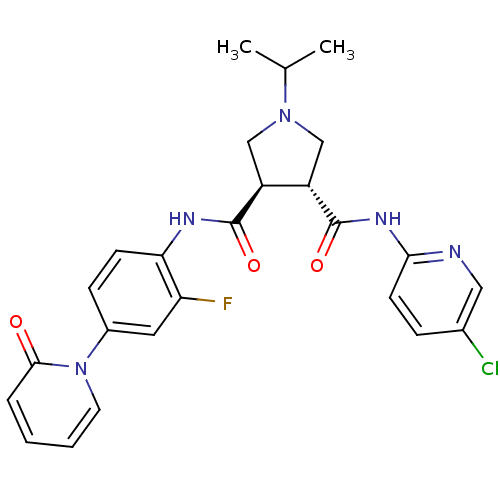
((3R,4R)-N3-(5-chloropyridin-2-yl)-N4-(2-fluoro-4-(...)Show SMILES CC(C)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C25H25ClFN5O3/c1-15(2)31-13-18(19(14-31)25(35)30-22-9-6-16(26)12-28-22)24(34)29-21-8-7-17(11-20(21)27)32-10-4-3-5-23(32)33/h3-12,15,18-19H,13-14H2,1-2H3,(H,29,34)(H,28,30,35)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324746
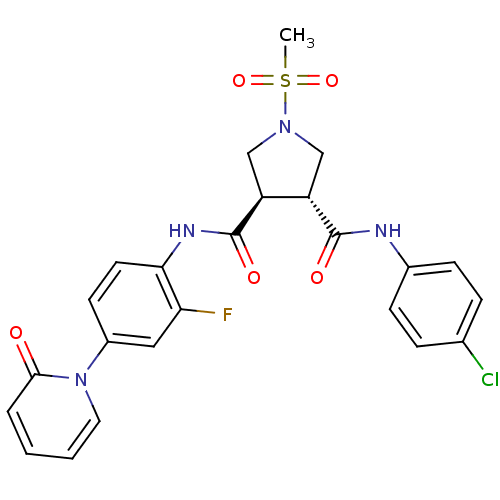
((3R,4R)-1-METHANESULFONYL-PYRROLIDINE-3,4-DICARBOX...)Show SMILES CS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H22ClFN4O5S/c1-36(34,35)29-13-18(23(32)27-16-7-5-15(25)6-8-16)19(14-29)24(33)28-21-10-9-17(12-20(21)26)30-11-3-2-4-22(30)31/h2-12,18-19H,13-14H2,1H3,(H,27,32)(H,28,33)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324789
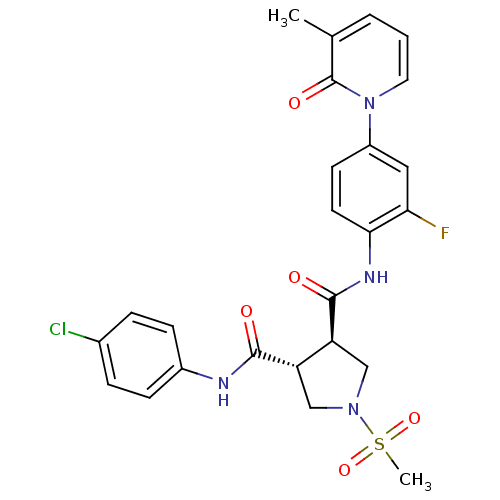
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(3-meth...)Show SMILES Cc1cccn(-c2ccc(NC(=O)[C@H]3CN(C[C@@H]3C(=O)Nc3ccc(Cl)cc3)S(C)(=O)=O)c(F)c2)c1=O |r| Show InChI InChI=1S/C25H24ClFN4O5S/c1-15-4-3-11-31(25(15)34)18-9-10-22(21(27)12-18)29-24(33)20-14-30(37(2,35)36)13-19(20)23(32)28-17-7-5-16(26)6-8-17/h3-12,19-20H,13-14H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035535
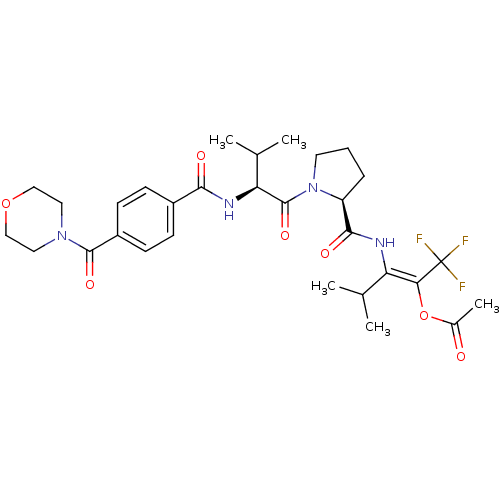
(Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)N\C(C(C)C)=C(\OC(C)=O)C(F)(F)F Show InChI InChI=1S/C30H39F3N4O7/c1-17(2)23(25(30(31,32)33)44-19(5)38)34-27(40)22-7-6-12-37(22)29(42)24(18(3)4)35-26(39)20-8-10-21(11-9-20)28(41)36-13-15-43-16-14-36/h8-11,17-18,22,24H,6-7,12-16H2,1-5H3,(H,34,40)(H,35,39)/b25-23+/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324753
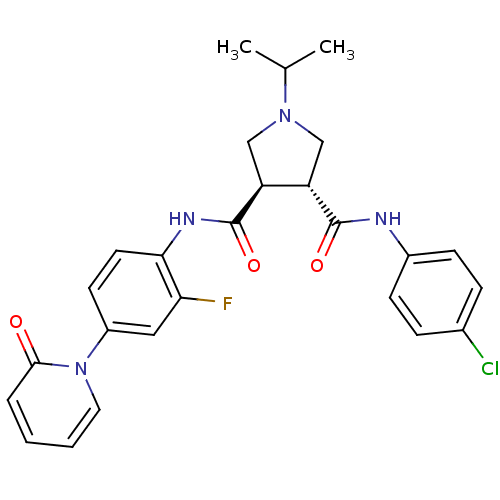
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES CC(C)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H26ClFN4O3/c1-16(2)31-14-20(25(34)29-18-8-6-17(27)7-9-18)21(15-31)26(35)30-23-11-10-19(13-22(23)28)32-12-4-3-5-24(32)33/h3-13,16,20-21H,14-15H2,1-2H3,(H,29,34)(H,30,35)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50324745
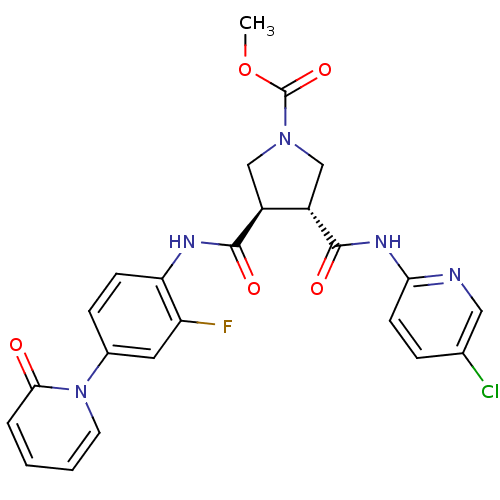
((3R,4R)-methyl 3-(5-chloropyridin-2-ylcarbamoyl)-4...)Show SMILES COC(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C24H21ClFN5O5/c1-36-24(35)30-12-16(17(13-30)23(34)29-20-8-5-14(25)11-27-20)22(33)28-19-7-6-15(10-18(19)26)31-9-3-2-4-21(31)32/h2-11,16-17H,12-13H2,1H3,(H,28,33)(H,27,29,34)/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324756
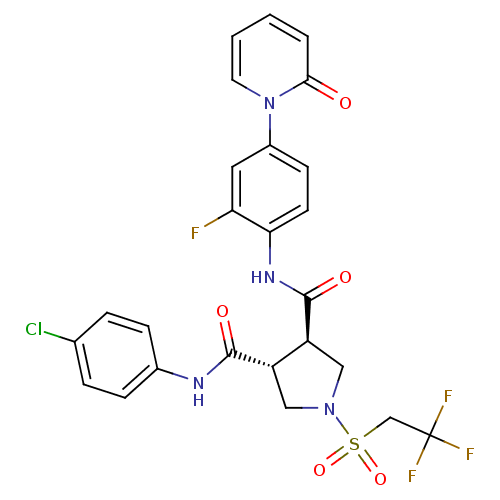
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES Fc1cc(ccc1NC(=O)[C@H]1CN(C[C@@H]1C(=O)Nc1ccc(Cl)cc1)S(=O)(=O)CC(F)(F)F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H21ClF4N4O5S/c26-15-4-6-16(7-5-15)31-23(36)18-12-33(40(38,39)14-25(28,29)30)13-19(18)24(37)32-21-9-8-17(11-20(21)27)34-10-2-1-3-22(34)35/h1-11,18-19H,12-14H2,(H,31,36)(H,32,37)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324762
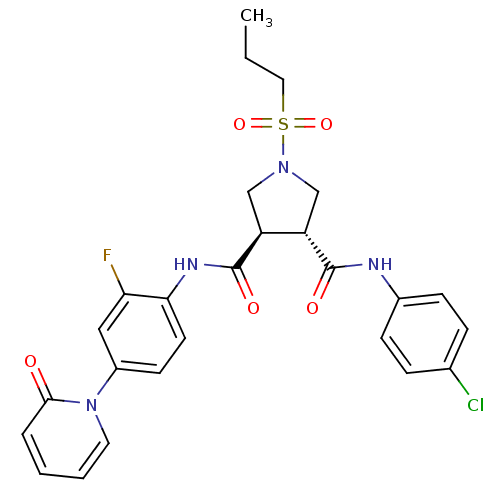
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES CCCS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H26ClFN4O5S/c1-2-13-38(36,37)31-15-20(25(34)29-18-8-6-17(27)7-9-18)21(16-31)26(35)30-23-11-10-19(14-22(23)28)32-12-4-3-5-24(32)33/h3-12,14,20-21H,2,13,15-16H2,1H3,(H,29,34)(H,30,35)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035492
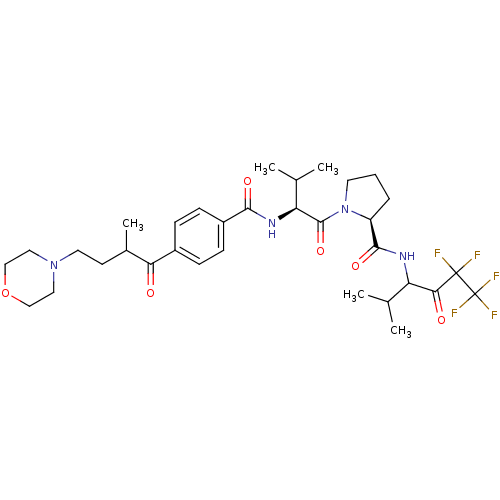
((S)-1-{(S)-3-Methyl-2-[4-(2-methyl-4-morpholin-4-y...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)C(C)CCN1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C33H45F5N4O6/c1-19(2)25(28(44)32(34,35)33(36,37)38)39-30(46)24-7-6-13-42(24)31(47)26(20(3)4)40-29(45)23-10-8-22(9-11-23)27(43)21(5)12-14-41-15-17-48-18-16-41/h8-11,19-21,24-26H,6-7,12-18H2,1-5H3,(H,39,46)(H,40,45)/t21?,24-,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324769
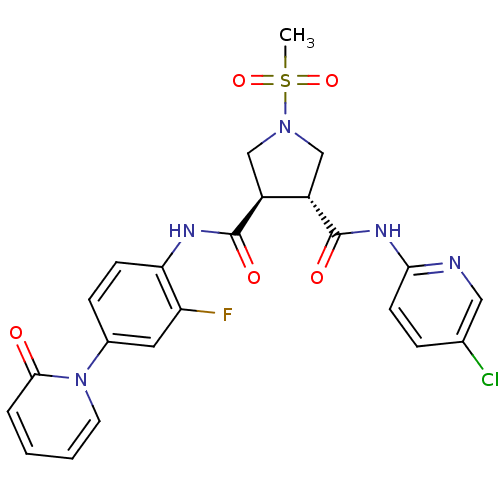
((3R,4R)-N3-(5-chloropyridin-2-yl)-N4-(2-fluoro-4-(...)Show SMILES CS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C23H21ClFN5O5S/c1-36(34,35)29-12-16(17(13-29)23(33)28-20-8-5-14(24)11-26-20)22(32)27-19-7-6-15(10-18(19)25)30-9-3-2-4-21(30)31/h2-11,16-17H,12-13H2,1H3,(H,27,32)(H,26,28,33)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037812
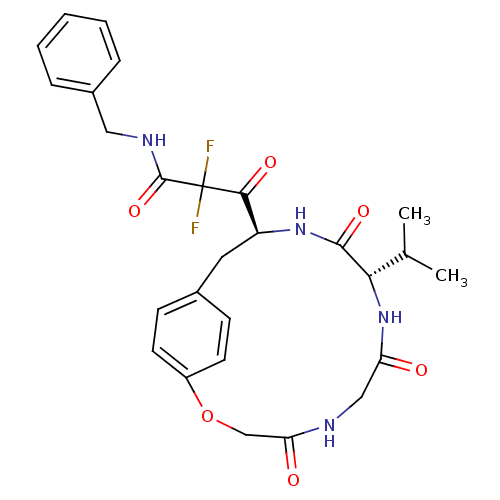
(CHEMBL331994 | MDL-104168 | N-Benzyl-2,2-difluoro-...)Show SMILES CC(C)[C@@H]1NC(=O)CNC(=O)COc2ccc(C[C@H](NC1=O)C(=O)C(F)(F)C(=O)NCc1ccccc1)cc2 Show InChI InChI=1S/C27H30F2N4O6/c1-16(2)23-25(37)32-20(24(36)27(28,29)26(38)31-13-18-6-4-3-5-7-18)12-17-8-10-19(11-9-17)39-15-22(35)30-14-21(34)33-23/h3-11,16,20,23H,12-15H2,1-2H3,(H,30,35)(H,31,38)(H,32,37)(H,33,34)/t20-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the ability to inhibit HIV-protease. |
J Med Chem 37: 3684-92 (1994)
BindingDB Entry DOI: 10.7270/Q2ZW1JZT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324764
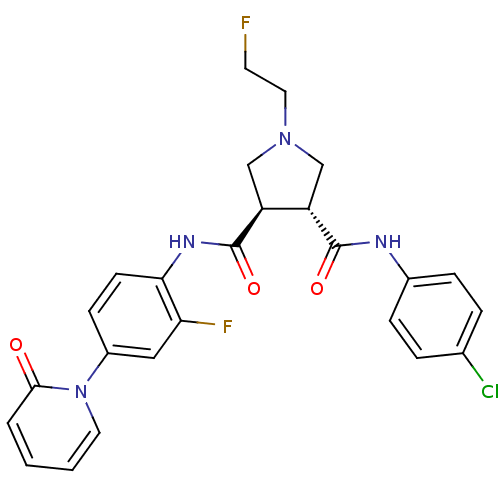
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES FCCN1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H23ClF2N4O3/c26-16-4-6-17(7-5-16)29-24(34)19-14-31(12-10-27)15-20(19)25(35)30-22-9-8-18(13-21(22)28)32-11-2-1-3-23(32)33/h1-9,11,13,19-20H,10,12,14-15H2,(H,29,34)(H,30,35)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035494
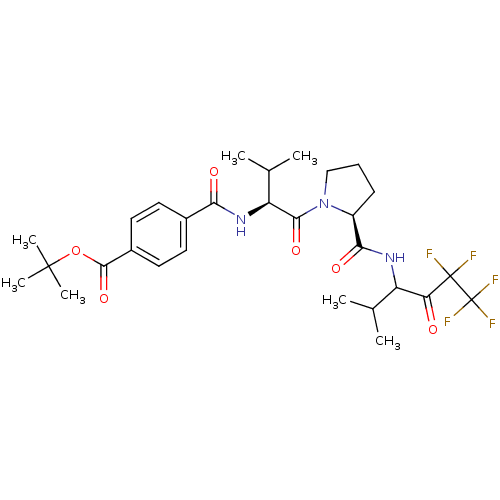
(CHEMBL429359 | N-{(S)-2-Methyl-1-[(S)-2-(3,3,4,4,4...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)OC(C)(C)C)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C29H38F5N3O6/c1-15(2)20(22(38)28(30,31)29(32,33)34)35-24(40)19-9-8-14-37(19)25(41)21(16(3)4)36-23(39)17-10-12-18(13-11-17)26(42)43-27(5,6)7/h10-13,15-16,19-21H,8-9,14H2,1-7H3,(H,35,40)(H,36,39)/t19-,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324751
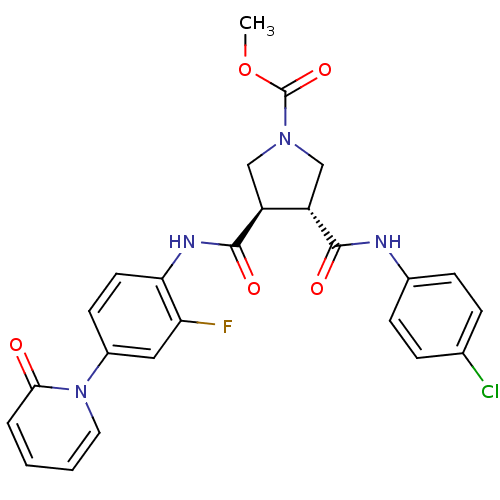
((3R,4R)-methyl 3-(4-chlorophenylcarbamoyl)-4-(2-fl...)Show SMILES COC(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H22ClFN4O5/c1-36-25(35)30-13-18(23(33)28-16-7-5-15(26)6-8-16)19(14-30)24(34)29-21-10-9-17(12-20(21)27)31-11-3-2-4-22(31)32/h2-12,18-19H,13-14H2,1H3,(H,28,33)(H,29,34)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324792
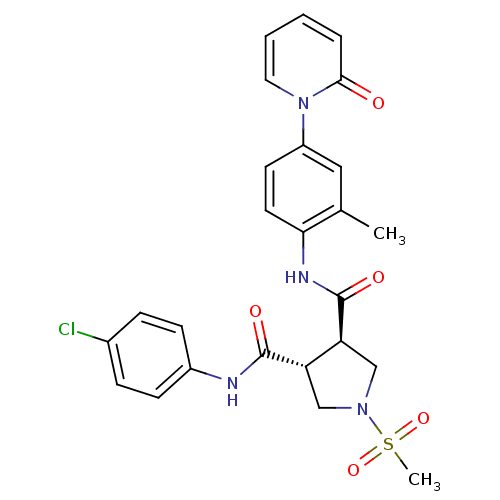
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-methyl-4-(2-oxop...)Show SMILES Cc1cc(ccc1NC(=O)[C@H]1CN(C[C@@H]1C(=O)Nc1ccc(Cl)cc1)S(C)(=O)=O)-n1ccccc1=O |r| Show InChI InChI=1S/C25H25ClN4O5S/c1-16-13-19(30-12-4-3-5-23(30)31)10-11-22(16)28-25(33)21-15-29(36(2,34)35)14-20(21)24(32)27-18-8-6-17(26)7-9-18/h3-13,20-21H,14-15H2,1-2H3,(H,27,32)(H,28,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324795
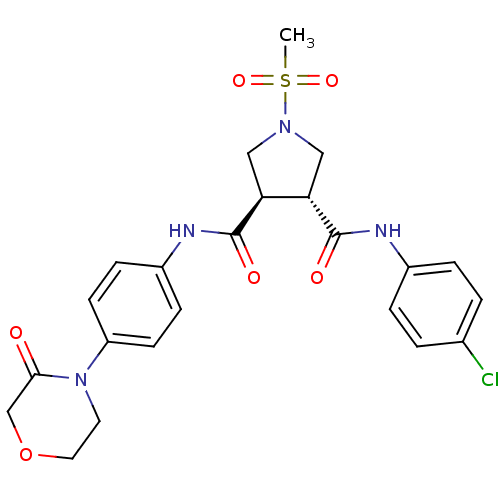
((3R,4R)-N3-(4-chlorophenyl)-1-(methylsulfonyl)-N4-...)Show SMILES CS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1)N1CCOCC1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H25ClN4O6S/c1-35(32,33)27-12-19(22(30)25-16-4-2-15(24)3-5-16)20(13-27)23(31)26-17-6-8-18(9-7-17)28-10-11-34-14-21(28)29/h2-9,19-20H,10-14H2,1H3,(H,25,30)(H,26,31)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035485
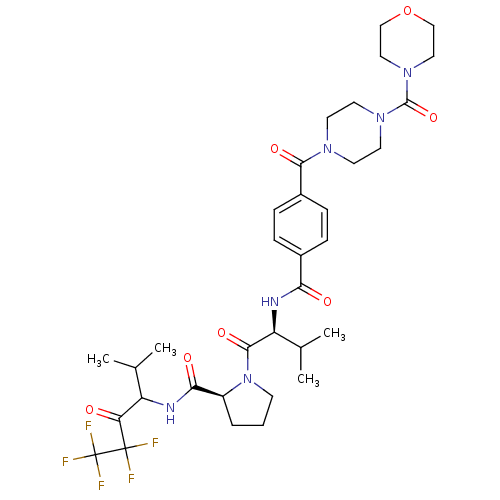
((S)-1-((S)-3-Methyl-2-{4-[4-(morpholine-4-carbonyl...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCN(CC1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C34H45F5N6O7/c1-20(2)25(27(46)33(35,36)34(37,38)39)40-29(48)24-6-5-11-45(24)31(50)26(21(3)4)41-28(47)22-7-9-23(10-8-22)30(49)42-12-14-43(15-13-42)32(51)44-16-18-52-19-17-44/h7-10,20-21,24-26H,5-6,11-19H2,1-4H3,(H,40,48)(H,41,47)/t24-,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324750
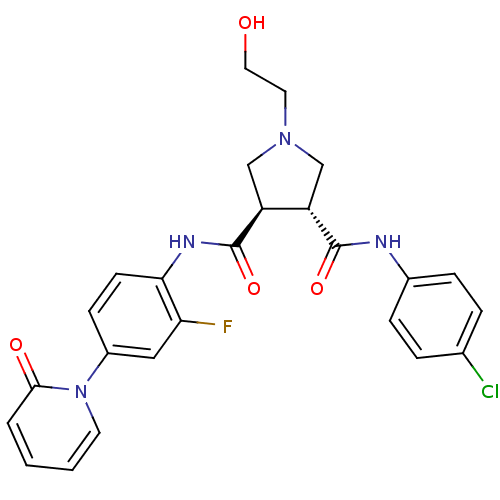
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES OCCN1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H24ClFN4O4/c26-16-4-6-17(7-5-16)28-24(34)19-14-30(11-12-32)15-20(19)25(35)29-22-9-8-18(13-21(22)27)31-10-2-1-3-23(31)33/h1-10,13,19-20,32H,11-12,14-15H2,(H,28,34)(H,29,35)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324742
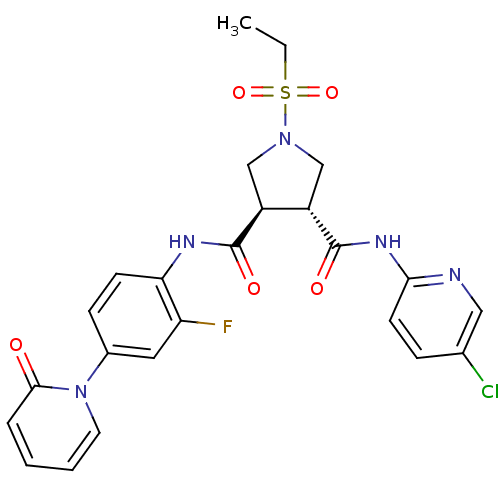
((3R,4R)-N3-(5-chloropyridin-2-yl)-1-(ethylsulfonyl...)Show SMILES CCS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C24H23ClFN5O5S/c1-2-37(35,36)30-13-17(18(14-30)24(34)29-21-9-6-15(25)12-27-21)23(33)28-20-8-7-16(11-19(20)26)31-10-4-3-5-22(31)32/h3-12,17-18H,2,13-14H2,1H3,(H,28,33)(H,27,29,34)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035495
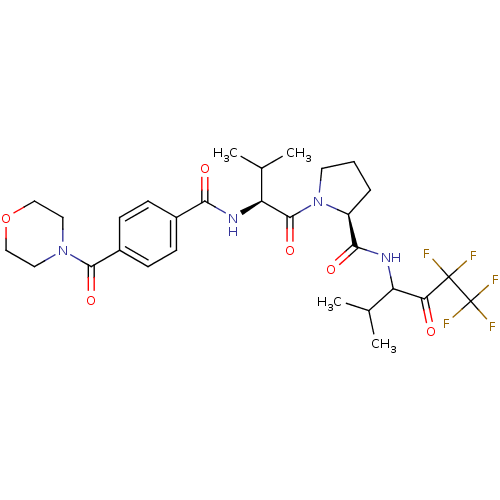
((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C29H37F5N4O6/c1-16(2)21(23(39)28(30,31)29(32,33)34)35-25(41)20-6-5-11-38(20)27(43)22(17(3)4)36-24(40)18-7-9-19(10-8-18)26(42)37-12-14-44-15-13-37/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,35,41)(H,36,40)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 50 mg/kg |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035532
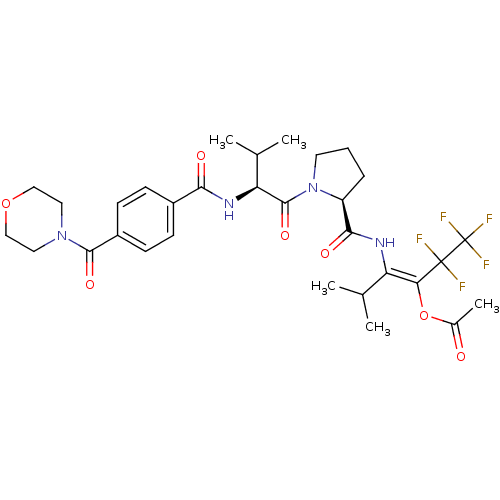
(Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)N\C(C(C)C)=C(\OC(C)=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C31H39F5N4O7/c1-17(2)23(25(47-19(5)41)30(32,33)31(34,35)36)37-27(43)22-7-6-12-40(22)29(45)24(18(3)4)38-26(42)20-8-10-21(11-9-20)28(44)39-13-15-46-16-14-39/h8-11,17-18,22,24H,6-7,12-16H2,1-5H3,(H,37,43)(H,38,42)/b25-23+/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035495

((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C29H37F5N4O6/c1-16(2)21(23(39)28(30,31)29(32,33)34)35-25(41)20-6-5-11-38(20)27(43)22(17(3)4)36-24(40)18-7-9-19(10-8-18)26(42)37-12-14-44-15-13-37/h7-10,16-17,20-22H,5-6,11-15H2,1-4H3,(H,35,41)(H,36,40)/t20-,21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035531
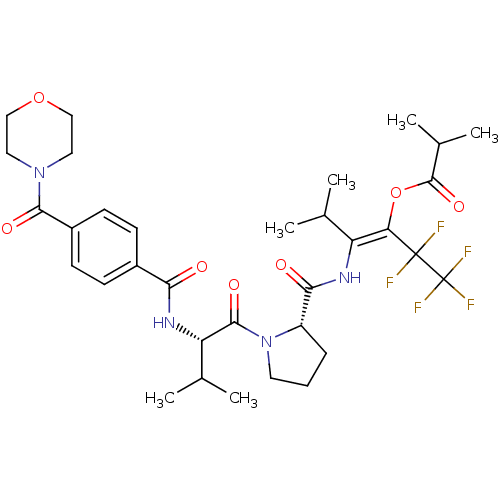
(CHEMBL74332 | Isobutyric acid (E)-3-methyl-2-[((S)...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)N\C(C(C)C)=C(\OC(=O)C(C)C)C(F)(F)C(F)(F)F Show InChI InChI=1S/C33H43F5N4O7/c1-18(2)24(26(49-31(47)20(5)6)32(34,35)33(36,37)38)39-28(44)23-8-7-13-42(23)30(46)25(19(3)4)40-27(43)21-9-11-22(12-10-21)29(45)41-14-16-48-17-15-41/h9-12,18-20,23,25H,7-8,13-17H2,1-6H3,(H,39,44)(H,40,43)/b26-24+/t23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035530
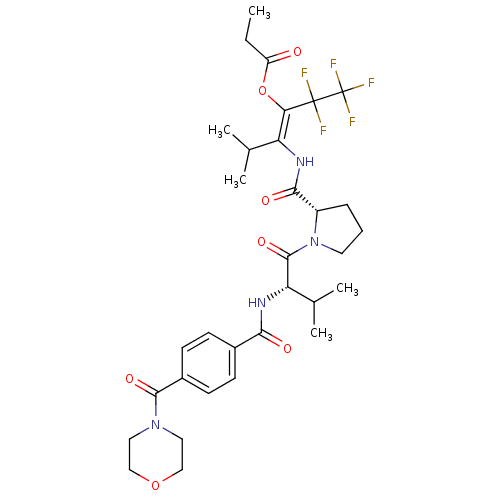
(CHEMBL74651 | Propionic acid (E)-3-methyl-2-[((S)-...)Show SMILES CCC(=O)O\C(=C(\NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)c1ccc(cc1)C(=O)N1CCOCC1)C(C)C)C(C)C)C(F)(F)C(F)(F)F Show InChI InChI=1S/C32H41F5N4O7/c1-6-23(42)48-26(31(33,34)32(35,36)37)24(18(2)3)38-28(44)22-8-7-13-41(22)30(46)25(19(4)5)39-27(43)20-9-11-21(12-10-20)29(45)40-14-16-47-17-15-40/h9-12,18-19,22,25H,6-8,13-17H2,1-5H3,(H,38,44)(H,39,43)/b26-24+/t22-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. |
J Med Chem 38: 223-33 (1995)
BindingDB Entry DOI: 10.7270/Q2CC0ZPC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324745

((3R,4R)-methyl 3-(5-chloropyridin-2-ylcarbamoyl)-4...)Show SMILES COC(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C24H21ClFN5O5/c1-36-24(35)30-12-16(17(13-30)23(34)29-20-8-5-14(25)11-27-20)22(33)28-19-7-6-15(10-18(19)26)31-9-3-2-4-21(31)32/h2-11,16-17H,12-13H2,1H3,(H,28,33)(H,27,29,34)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324745

((3R,4R)-methyl 3-(5-chloropyridin-2-ylcarbamoyl)-4...)Show SMILES COC(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cn1 |r| Show InChI InChI=1S/C24H21ClFN5O5/c1-36-24(35)30-12-16(17(13-30)23(34)29-20-8-5-14(25)11-27-20)22(33)28-19-7-6-15(10-18(19)26)31-9-3-2-4-21(31)32/h2-11,16-17H,12-13H2,1H3,(H,28,33)(H,27,29,34)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035487
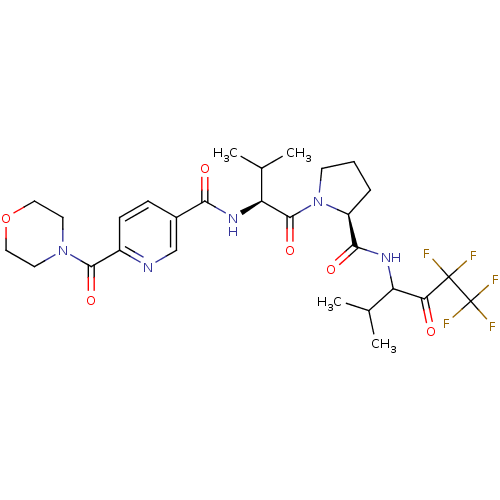
(CHEMBL140639 | N-{(S)-2-Methyl-1-[(S)-2-(3,3,4,4,4...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(nc1)C(=O)N1CCOCC1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C28H36F5N5O6/c1-15(2)20(22(39)27(29,30)28(31,32)33)35-24(41)19-6-5-9-38(19)26(43)21(16(3)4)36-23(40)17-7-8-18(34-14-17)25(42)37-10-12-44-13-11-37/h7-8,14-16,19-21H,5-6,9-13H2,1-4H3,(H,35,41)(H,36,40)/t19-,20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324748
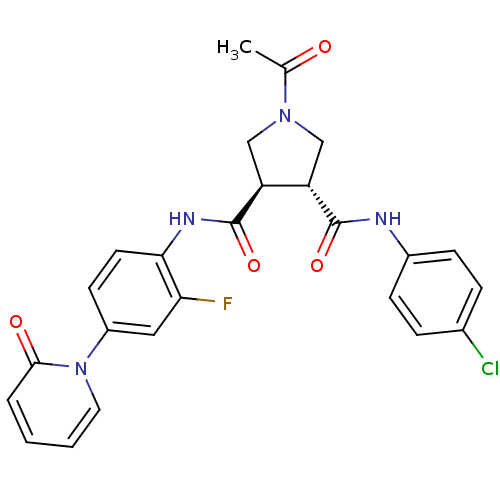
((3R,4R)-1-acetyl-N3-(4-chlorophenyl)-N4-(2-fluoro-...)Show SMILES CC(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H22ClFN4O4/c1-15(32)30-13-19(24(34)28-17-7-5-16(26)6-8-17)20(14-30)25(35)29-22-10-9-18(12-21(22)27)31-11-3-2-4-23(31)33/h2-12,19-20H,13-14H2,1H3,(H,28,34)(H,29,35)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035502
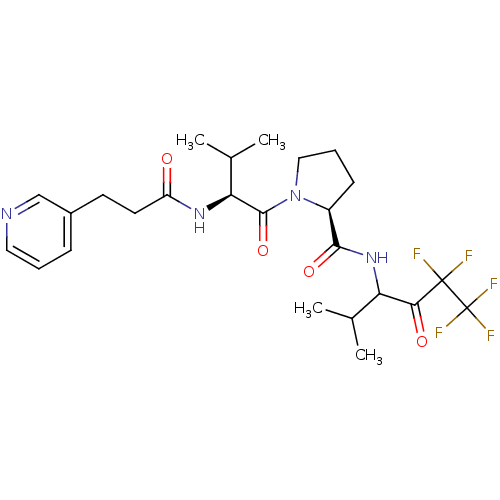
((S)-1-[(S)-3-Methyl-2-(3-pyridin-3-yl-propionylami...)Show SMILES CC(C)[C@H](NC(=O)CCc1cccnc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)C(F)(F)F Show InChI InChI=1S/C25H33F5N4O4/c1-14(2)19(21(36)24(26,27)25(28,29)30)33-22(37)17-8-6-12-34(17)23(38)20(15(3)4)32-18(35)10-9-16-7-5-11-31-13-16/h5,7,11,13-15,17,19-20H,6,8-10,12H2,1-4H3,(H,32,35)(H,33,37)/t17-,19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of human neutrophil elastase |
J Med Chem 37: 4538-53 (1995)
BindingDB Entry DOI: 10.7270/Q28P615X |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324784
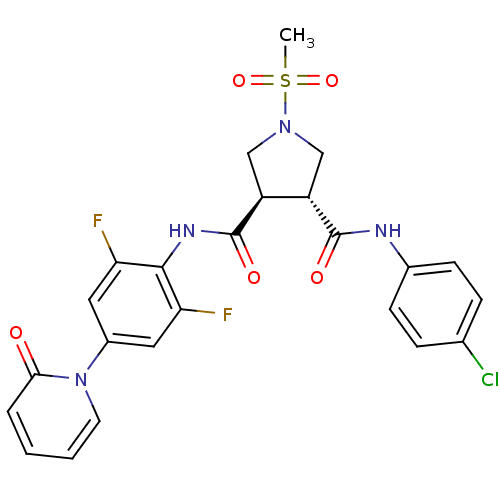
((3R,4R)-N3-(4-chlorophenyl)-N4-(2,6-difluoro-4-(2-...)Show SMILES CS(=O)(=O)N1C[C@@H]([C@H](C1)C(=O)Nc1c(F)cc(cc1F)-n1ccccc1=O)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H21ClF2N4O5S/c1-37(35,36)30-12-17(23(33)28-15-7-5-14(25)6-8-15)18(13-30)24(34)29-22-19(26)10-16(11-20(22)27)31-9-3-2-4-21(31)32/h2-11,17-18H,12-13H2,1H3,(H,28,33)(H,29,34)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50324765
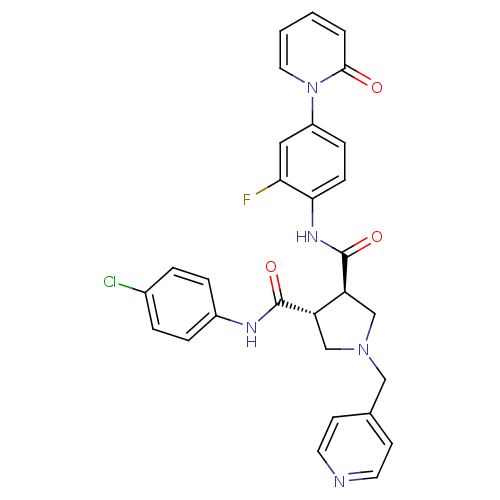
((3R,4R)-N3-(4-chlorophenyl)-N4-(2-fluoro-4-(2-oxop...)Show SMILES Fc1cc(ccc1NC(=O)[C@H]1CN(Cc2ccncc2)C[C@@H]1C(=O)Nc1ccc(Cl)cc1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H25ClFN5O3/c30-20-4-6-21(7-5-20)33-28(38)23-17-35(16-19-10-12-32-13-11-19)18-24(23)29(39)34-26-9-8-22(15-25(26)31)36-14-2-1-3-27(36)37/h1-15,23-24H,16-18H2,(H,33,38)(H,34,39)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to factor 10a |
Bioorg Med Chem Lett 20: 5313-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.126
BindingDB Entry DOI: 10.7270/Q2542NSG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data