

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27614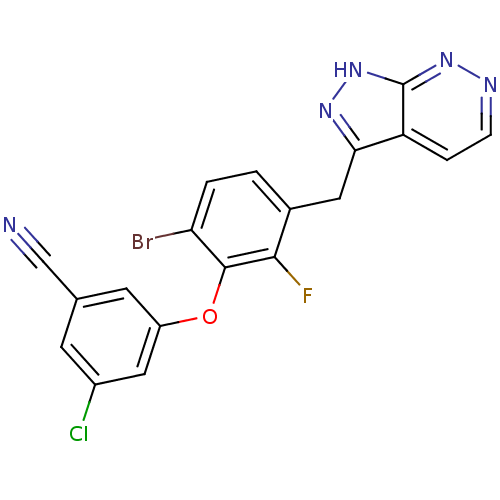 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | n/a | n/a | 3 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27614 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | >25 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 2 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27612 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | 4 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27613 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | 18 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27611 (3-[6-bromo-2-fluoro-3-({6-methyl-7-oxo-1H,6H,7H-py...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 97 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27615 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyrazin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | >100 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27609 (3-{[4-bromo-3-(3-chloro-5-cyanophenoxy)-2-fluoroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | 53 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27610 (3-{3-[(7-amino-1H-indazol-3-yl)methyl]-6-bromo-2-f...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | 84 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27609 (3-{[4-bromo-3-(3-chloro-5-cyanophenoxy)-2-fluoroph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 5 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27610 (3-{3-[(7-amino-1H-indazol-3-yl)methyl]-6-bromo-2-f...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 8 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50262376 (6-((7-(3-chlorobenzyl)benzofuran-5-yl)methyl)pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50261746 (3-chloro-5-(6-chloro-2-fluoro-3-((6-methyl-5-oxo-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4348-51 (2008) Article DOI: 10.1016/j.bmcl.2008.06.080 BindingDB Entry DOI: 10.7270/Q2ZK5GG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50262377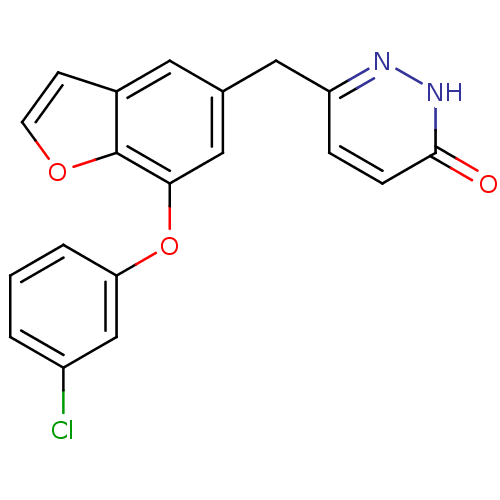 (6-((7-(3-chlorophenoxy)benzofuran-5-yl)methyl)pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50262375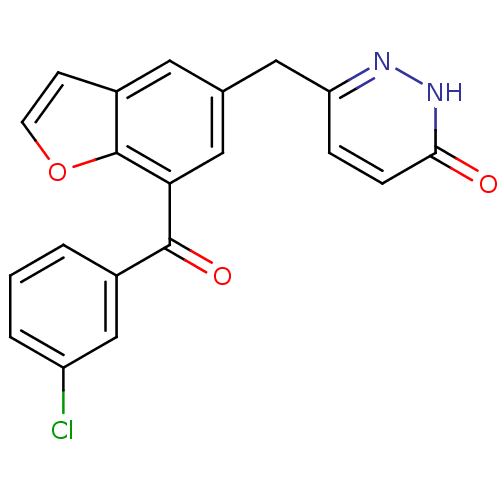 (6-((7-(3-chlorobenzoyl)benzofuran-5-yl)methyl)pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50262487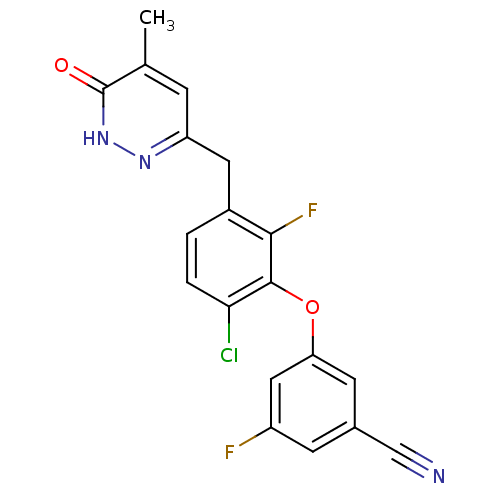 (3-(6-chloro-2-fluoro-3-((5-methyl-6-oxo-1,6-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50261877 (3-chloro-5-(2-fluoro-6-methyl-3-((4-methyl-5-oxo-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4348-51 (2008) Article DOI: 10.1016/j.bmcl.2008.06.080 BindingDB Entry DOI: 10.7270/Q2ZK5GG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50262487 (3-(6-chloro-2-fluoro-3-((5-methyl-6-oxo-1,6-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50262487 (3-(6-chloro-2-fluoro-3-((5-methyl-6-oxo-1,6-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of recombinant CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262487 (3-(6-chloro-2-fluoro-3-((5-methyl-6-oxo-1,6-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50262487 (3-(6-chloro-2-fluoro-3-((5-methyl-6-oxo-1,6-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 18: 4352-4 (2008) Article DOI: 10.1016/j.bmcl.2008.06.072 BindingDB Entry DOI: 10.7270/Q2XK8FC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27612 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27617 (5-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27616 (3-chloro-5-(6-chloro-2-fluoro-3-{1H-pyrazolo[3,4-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27618 (3-(2-bromo-4-{1H-pyrazolo[3,4-c]pyridazin-3-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | >100 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27617 (5-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K691N,Y769C]/[588-1147,K691N,Y769C] (Human immunodeficiency virus type 1) | BDBM27616 (3-chloro-5-(6-chloro-2-fluoro-3-{1H-pyrazolo[3,4-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27618 (3-(2-bromo-4-{1H-pyrazolo[3,4-c]pyridazin-3-ylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||