

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cytochrome P450 1A2 | ||
| Ligand | BDBM50336315 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_716613 (CHEMBL1670392) | ||
| IC50 | >20000±n/a nM | ||
| Citation |  Jin, M; Kleinberg, A; Cooke, A; Gokhale, PC; Foreman, K; Dong, H; Siu, KW; Bittner, MA; Mulvihill, KM; Yao, Y; Landfair, D; O'Connor, M; Mak, G; Pachter, JA; Wild, R; Rosenfeld-Franklin, M; Ji, Q; Mulvihill, MJ Potent and selective cyclohexyl-derived imidazopyrazine insulin-like growth factor 1 receptor inhibitors with in vivo efficacy. Bioorg Med Chem Lett21:1176-80 (2011) [PubMed] Article Jin, M; Kleinberg, A; Cooke, A; Gokhale, PC; Foreman, K; Dong, H; Siu, KW; Bittner, MA; Mulvihill, KM; Yao, Y; Landfair, D; O'Connor, M; Mak, G; Pachter, JA; Wild, R; Rosenfeld-Franklin, M; Ji, Q; Mulvihill, MJ Potent and selective cyclohexyl-derived imidazopyrazine insulin-like growth factor 1 receptor inhibitors with in vivo efficacy. Bioorg Med Chem Lett21:1176-80 (2011) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Cytochrome P450 1A2 | |||
| Name: | Cytochrome P450 1A2 | ||
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 58423.38 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P05177 | ||
| Residue: | 516 | ||
| Sequence: |
| ||
| BDBM50336315 | |||
| n/a | |||
| Name | BDBM50336315 | ||
| Synonyms: | CHEMBL1667948 | trans-4-(8-amino-1-(8-fluoro-2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl)-N-(1H-benzo[d]imidazol-2-yl)cyclohexanecarboxamide | ||
| Type | Small organic molecule | ||
| Emp. Form. | C35H29FN8O | ||
| Mol. Mass. | 596.6562 | ||
| SMILES | Nc1nccn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccccc2[nH]1 |r,wU:27.31,wD:30.38,(-9.22,-32.11,;-9.21,-33.66,;-10.54,-34.43,;-10.55,-35.97,;-9.21,-36.74,;-7.87,-35.96,;-6.4,-36.43,;-5.5,-35.18,;-6.41,-33.93,;-5.64,-32.61,;-6.41,-31.28,;-5.64,-29.95,;-4.09,-29.94,;-3.33,-28.62,;-1.8,-28.61,;-1.03,-29.95,;-1.8,-31.28,;-3.33,-31.27,;-4.1,-32.61,;-3.33,-33.95,;.51,-29.95,;1.28,-31.28,;2.82,-31.28,;3.59,-29.95,;2.81,-28.61,;1.27,-28.62,;-7.88,-34.42,;-5.91,-37.9,;-4.4,-38.2,;-3.92,-39.67,;-4.95,-40.82,;-6.46,-40.5,;-6.94,-39.04,;-4.47,-42.28,;-5.26,-43.61,;-2.93,-42.3,;-2.15,-40.97,;-2.75,-39.55,;-1.6,-38.53,;-1.58,-37,;-.25,-36.25,;1.08,-37.03,;1.06,-38.56,;-.27,-39.31,;-.61,-40.82,)| | ||
| Structure | 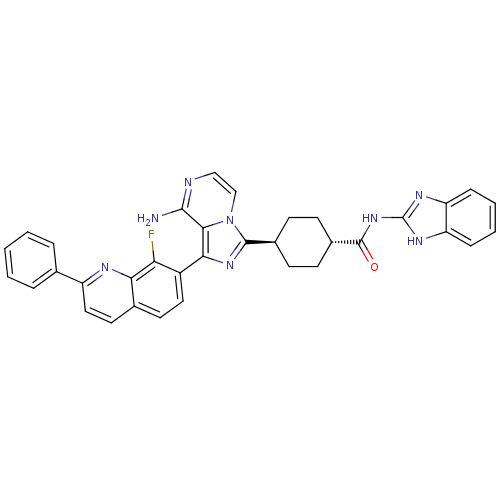
| ||