

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cytochrome P450 3A4 | ||
| Ligand | BDBM162157 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1509544 (CHEMBL3602291) | ||
| IC50 | >20000±n/a nM | ||
| Citation |  Holzer, P; Masuya, K; Furet, P; Kallen, J; Valat-Stachyra, T; Ferretti, S; Berghausen, J; Bouisset-Leonard, M; Buschmann, N; Pissot-Soldermann, C; Rynn, C; Ruetz, S; Stutz, S; Chène, P; Jeay, S; Gessier, F Discovery of a Dihydroisoquinolinone Derivative (NVP-CGM097): A Highly Potent and Selective MDM2 Inhibitor Undergoing Phase 1 Clinical Trials in p53wt Tumors. J Med Chem58:6348-58 (2015) [PubMed] Article Holzer, P; Masuya, K; Furet, P; Kallen, J; Valat-Stachyra, T; Ferretti, S; Berghausen, J; Bouisset-Leonard, M; Buschmann, N; Pissot-Soldermann, C; Rynn, C; Ruetz, S; Stutz, S; Chène, P; Jeay, S; Gessier, F Discovery of a Dihydroisoquinolinone Derivative (NVP-CGM097): A Highly Potent and Selective MDM2 Inhibitor Undergoing Phase 1 Clinical Trials in p53wt Tumors. J Med Chem58:6348-58 (2015) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Cytochrome P450 3A4 | |||
| Name: | Cytochrome P450 3A4 | ||
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 57349.57 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | n/a | ||
| Residue: | 503 | ||
| Sequence: |
| ||
| BDBM162157 | |||
| n/a | |||
| Name | BDBM162157 | ||
| Synonyms: | US9051279, 140 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C36H45ClN6O4 | ||
| Mol. Mass. | 661.233 | ||
| SMILES | COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1cnc(cn1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:36.43,wD:9.9,33.36,(12,4.23,;10.67,5,;9.34,4.23,;8,5,;6.67,4.23,;5.33,5,;4,4.23,;2.67,5,;4,2.69,;5.33,1.93,;5.33,.38,;4,-.38,;4,-1.93,;5.33,-2.69,;5.33,-4.23,;6.67,-1.93,;6.67,-.38,;6.67,2.69,;8,1.93,;9.34,2.69,;10.67,1.93,;10.67,.38,;12,-.38,;9.34,-.38,;2.67,1.93,;2.67,.38,;1.33,-.38,;,.38,;,1.93,;1.33,2.69,;-1.33,-.38,;-1.33,-1.93,;-2.67,.38,;-4,-.38,;-5.33,.38,;-6.67,-.38,;-6.67,-1.93,;-5.33,-2.69,;-4,-1.93,;-8,-2.69,;-8,-4.23,;-9.34,-5,;-10.67,-4.23,;-12,-5,;-10.67,-2.69,;-12,-1.93,;-9.34,-1.93,)| | ||
| Structure | 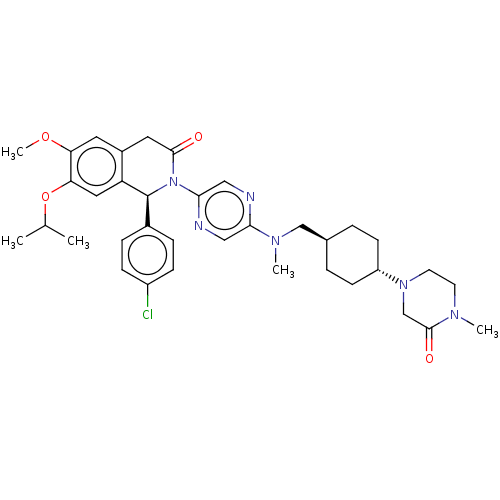
| ||