| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50111158 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1509935 (CHEMBL3607753) |
|---|
| IC50 | 3981±n/a nM |
|---|
| Citation |  Becherer, JD; Boros, EE; Carpenter, TY; Cowan, DJ; Deaton, DN; Haffner, CD; Jeune, MR; Kaldor, IW; Poole, JC; Preugschat, F; Rheault, TR; Schulte, CA; Shearer, BG; Shearer, TW; Shewchuk, LM; Smalley, TL; Stewart, EL; Stuart, JD; Ulrich, JC Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. J Med Chem58:7021-56 (2015) [PubMed] Article Becherer, JD; Boros, EE; Carpenter, TY; Cowan, DJ; Deaton, DN; Haffner, CD; Jeune, MR; Kaldor, IW; Poole, JC; Preugschat, F; Rheault, TR; Schulte, CA; Shearer, BG; Shearer, TW; Shewchuk, LM; Smalley, TL; Stewart, EL; Stuart, JD; Ulrich, JC Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. J Med Chem58:7021-56 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50111158 |
|---|
| n/a |
|---|
| Name | BDBM50111158 |
|---|
| Synonyms: | CHEMBL3604739 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H14Cl2FN3O |
|---|
| Mol. Mass. | 378.228 |
|---|
| SMILES | Cc1cc(NCc2c(F)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 |
|---|
| Structure | 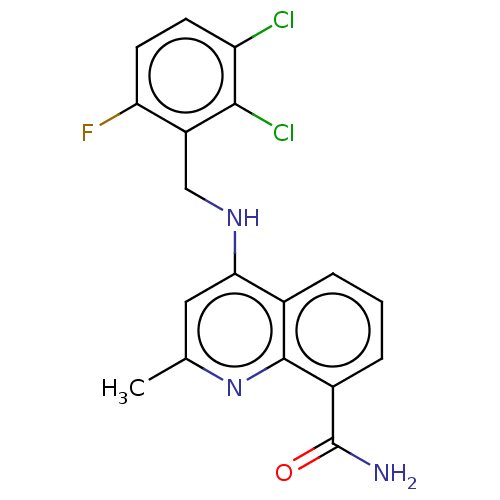
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Becherer, JD; Boros, EE; Carpenter, TY; Cowan, DJ; Deaton, DN; Haffner, CD; Jeune, MR; Kaldor, IW; Poole, JC; Preugschat, F; Rheault, TR; Schulte, CA; Shearer, BG; Shearer, TW; Shewchuk, LM; Smalley, TL; Stewart, EL; Stuart, JD; Ulrich, JC Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. J Med Chem58:7021-56 (2015) [PubMed] Article
Becherer, JD; Boros, EE; Carpenter, TY; Cowan, DJ; Deaton, DN; Haffner, CD; Jeune, MR; Kaldor, IW; Poole, JC; Preugschat, F; Rheault, TR; Schulte, CA; Shearer, BG; Shearer, TW; Shewchuk, LM; Smalley, TL; Stewart, EL; Stuart, JD; Ulrich, JC Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. J Med Chem58:7021-56 (2015) [PubMed] Article