| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50130505 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1525508 (CHEMBL3636861) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Elkamhawy, A; Viswanath, AN; Pae, AN; Kim, HY; Heo, JC; Park, WK; Lee, CO; Yang, H; Kim, KH; Nam, DH; Seol, HJ; Cho, H; Roh, EJ Discovery of potent and selective cytotoxic activity of new quinazoline-ureas against TMZ-resistant glioblastoma multiforme (GBM). Eur J Med Chem103:210-22 (2015) [PubMed] Article Elkamhawy, A; Viswanath, AN; Pae, AN; Kim, HY; Heo, JC; Park, WK; Lee, CO; Yang, H; Kim, KH; Nam, DH; Seol, HJ; Cho, H; Roh, EJ Discovery of potent and selective cytotoxic activity of new quinazoline-ureas against TMZ-resistant glioblastoma multiforme (GBM). Eur J Med Chem103:210-22 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50130505 |
|---|
| n/a |
|---|
| Name | BDBM50130505 |
|---|
| Synonyms: | CHEMBL3634101 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H19ClN4O2 |
|---|
| Mol. Mass. | 454.908 |
|---|
| SMILES | Clc1cccc(COc2cc3cncnc3cc2NC(=O)Nc2cccc3ccccc23)c1 |
|---|
| Structure | 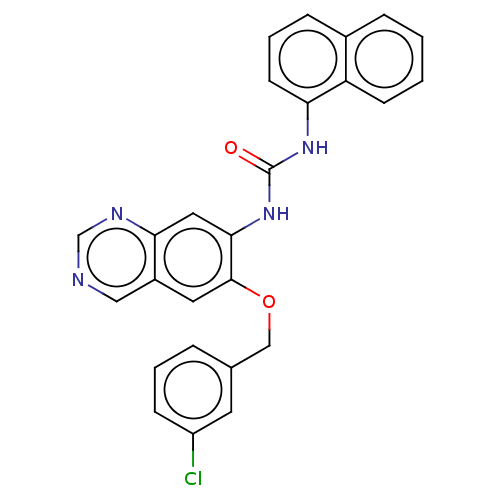
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Elkamhawy, A; Viswanath, AN; Pae, AN; Kim, HY; Heo, JC; Park, WK; Lee, CO; Yang, H; Kim, KH; Nam, DH; Seol, HJ; Cho, H; Roh, EJ Discovery of potent and selective cytotoxic activity of new quinazoline-ureas against TMZ-resistant glioblastoma multiforme (GBM). Eur J Med Chem103:210-22 (2015) [PubMed] Article
Elkamhawy, A; Viswanath, AN; Pae, AN; Kim, HY; Heo, JC; Park, WK; Lee, CO; Yang, H; Kim, KH; Nam, DH; Seol, HJ; Cho, H; Roh, EJ Discovery of potent and selective cytotoxic activity of new quinazoline-ureas against TMZ-resistant glioblastoma multiforme (GBM). Eur J Med Chem103:210-22 (2015) [PubMed] Article