 Found 14381 hits with Last Name = 'yang' and Initial = 'h'
Found 14381 hits with Last Name = 'yang' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124984
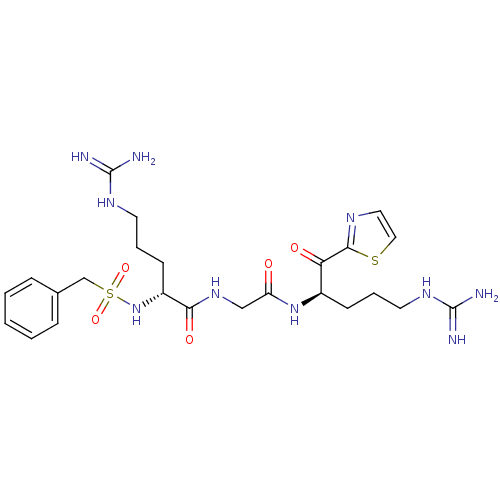
((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)33-19(35)14-32-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,34H,4-5,8-11,14-15H2,(H,32,37)(H,33,35)(H4,25,26,30)(H4,27,28,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599186
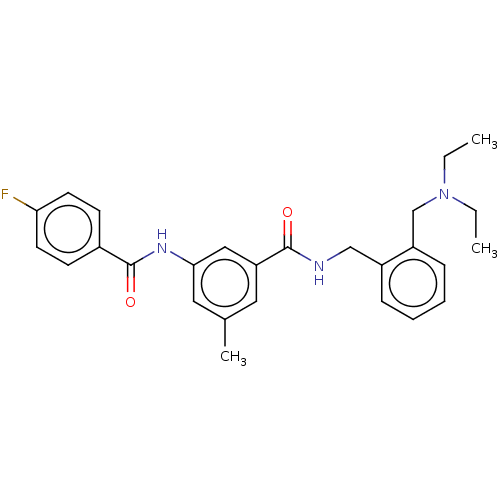
(CHEMBL5201089)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(C)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50124947
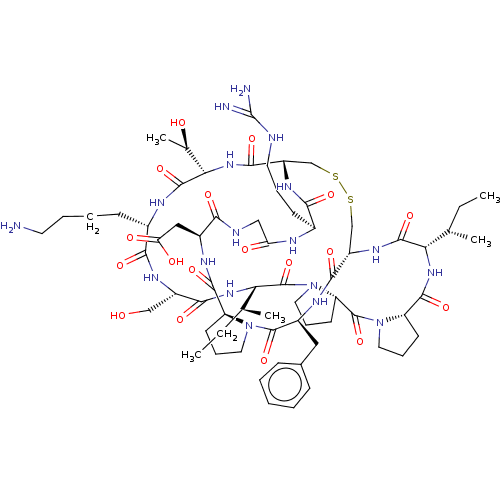
(CHEMBL453539)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of bovine beta-trypsin type-3 using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured over 60 mins |
J Med Chem 60: 504-510 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01011
BindingDB Entry DOI: 10.7270/Q2RJ4MRS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124975
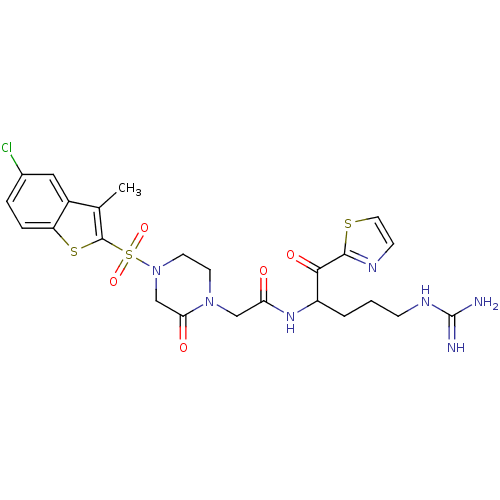
(2-[4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfon...)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)N1CCN(CC(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)C(=O)C1 Show InChI InChI=1S/C24H28ClN7O5S3/c1-14-16-11-15(25)4-5-18(16)39-23(14)40(36,37)32-9-8-31(20(34)13-32)12-19(33)30-17(3-2-6-29-24(26)27)21(35)22-28-7-10-38-22/h4-5,7,10-11,17H,2-3,6,8-9,12-13H2,1H3,(H,30,33)(H4,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50596723

(CHEMBL5205903 | US20230348421, Compound 59)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cn1)C(=O)NCc1ccc(OCC(F)(F)F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114246
BindingDB Entry DOI: 10.7270/Q20P142F |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50335638
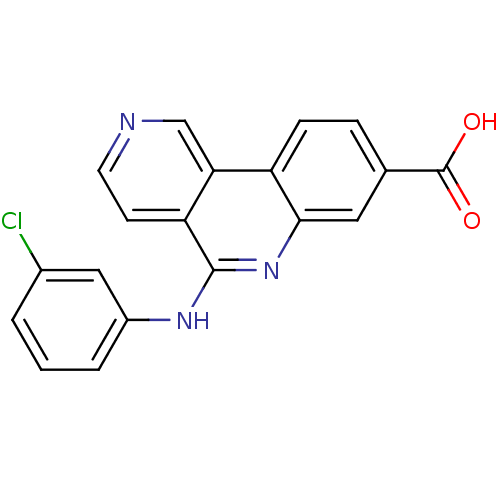
(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CK2alpha (unknown origin) using RRRADDSDDDDD as substrate in presence of [gamma33P]-ATP by autoradiography |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111581
BindingDB Entry DOI: 10.7270/Q29K4FJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599167
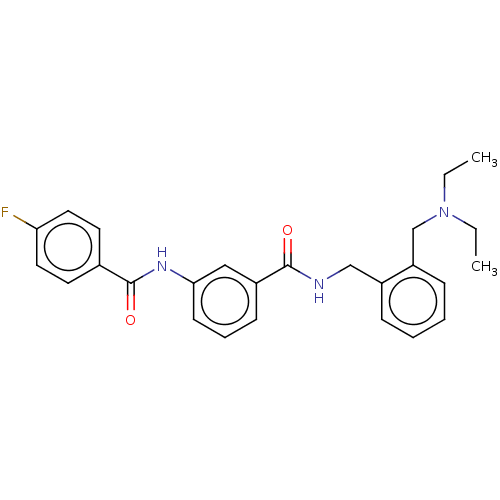
(CHEMBL5195228)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205
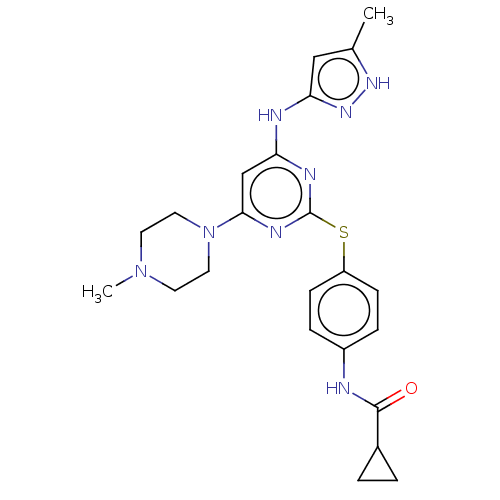
(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114424
BindingDB Entry DOI: 10.7270/Q2VX0MHV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50125017
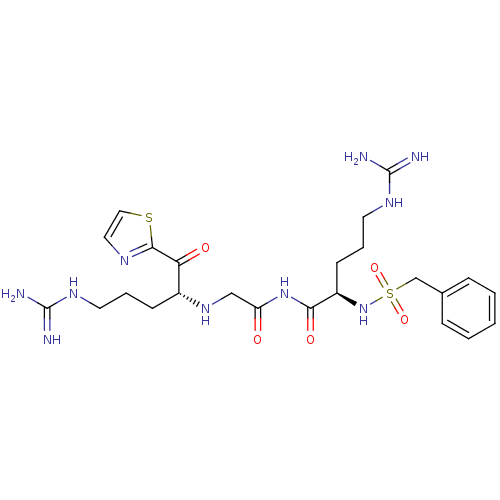
(CHEMBL350976 | N-((R)-5-Guanidino-2-phenylmethanes...)Show SMILES NC(=N)NCCC[C@@H](NCC(=O)NC(=O)[C@@H](CCCNC(N)=N)NS(=O)(=O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C24H36N10O5S2/c25-23(26)30-10-4-8-17(20(36)22-29-12-13-40-22)32-14-19(35)33-21(37)18(9-5-11-31-24(27)28)34-41(38,39)15-16-6-2-1-3-7-16/h1-3,6-7,12-13,17-18,32,34H,4-5,8-11,14-15H2,(H4,25,26,30)(H4,27,28,31)(H,33,35,37)/t17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against serine protease thrombin |
Bioorg Med Chem Lett 13: 729-32 (2003)
BindingDB Entry DOI: 10.7270/Q2T72GTS |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50373326
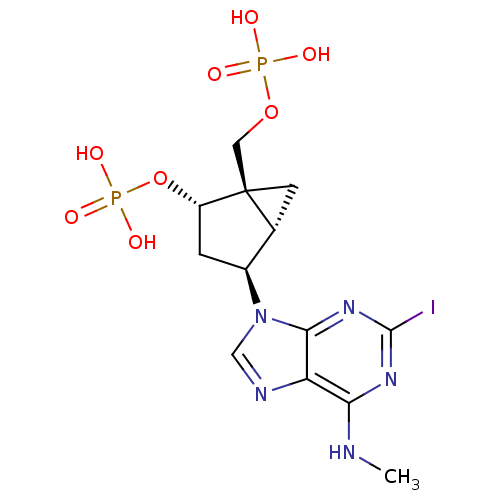
(CHEMBL444278)Show SMILES CNc1nc(I)nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12 Show InChI InChI=1S/C13H18IN5O8P2/c1-15-10-9-11(18-12(14)17-10)19(5-16-9)7-2-8(27-29(23,24)25)13(3-6(7)13)4-26-28(20,21)22/h5-8H,2-4H2,1H3,(H,15,17,18)(H2,20,21,22)(H2,23,24,25)/t6-,7+,8+,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]2-chloro-N 6- methyl-( N )-methanocarba-2'-deoxyadenosine 3 ' ,5 '-bis-phosphate from human P2Y1 expressed in baculovirus infecte... |
Eur J Med Chem 158: 302-310 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.014
BindingDB Entry DOI: 10.7270/Q27H1N8S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM10755
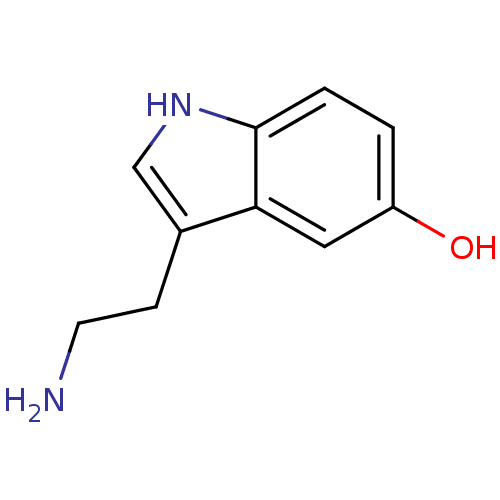
(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-HT1A receptor (unknown origin) expressed in HEK293 cells by liquid scintillation counter |
Bioorg Med Chem 21: 856-68 (2013)
Article DOI: 10.1016/j.bmc.2012.12.016
BindingDB Entry DOI: 10.7270/Q2JD4Z4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50244317
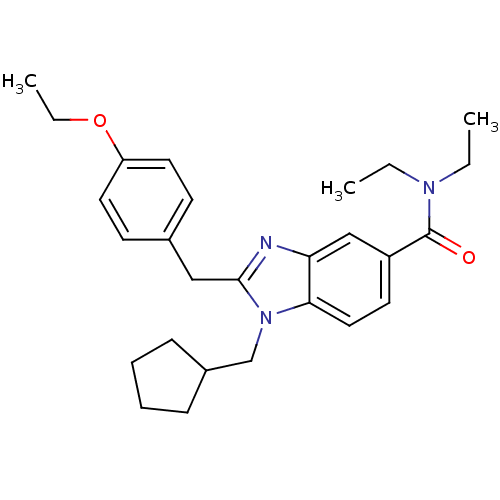
(2-(4-ethoxybenzyl)-1-(cyclopentylmethyl)-N,N-dieth...)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCC2)C(=O)N(CC)CC)cc1 Show InChI InChI=1S/C27H35N3O2/c1-4-29(5-2)27(31)22-13-16-25-24(18-22)28-26(30(25)19-21-9-7-8-10-21)17-20-11-14-23(15-12-20)32-6-3/h11-16,18,21H,4-10,17,19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3695-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.073
BindingDB Entry DOI: 10.7270/Q2J38SBN |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50124947

(CHEMBL453539)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of bovine beta-trypsin using N-t-Boc Gln-Ala-Arg-AMC as substrate preincubated with enzyme followed by substrate addition by Dixon plot an... |
J Med Chem 60: 504-510 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01011
BindingDB Entry DOI: 10.7270/Q2RJ4MRS |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from dopamine D2 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter |
Bioorg Med Chem 21: 856-68 (2013)
Article DOI: 10.1016/j.bmc.2012.12.016
BindingDB Entry DOI: 10.7270/Q2JD4Z4M |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247
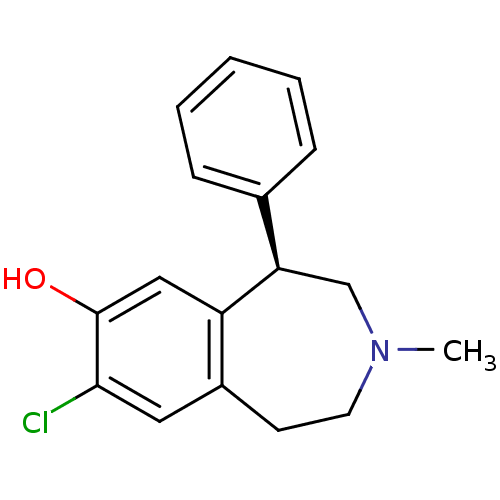
(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter |
Bioorg Med Chem 21: 856-68 (2013)
Article DOI: 10.1016/j.bmc.2012.12.016
BindingDB Entry DOI: 10.7270/Q2JD4Z4M |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124979
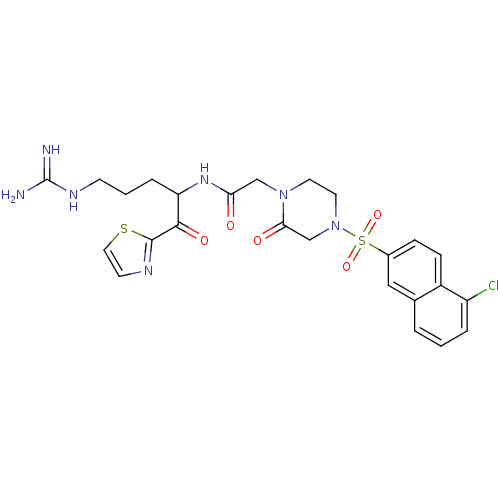
(2-[4-(5-Chloro-naphthalene-2-sulfonyl)-2-oxo-piper...)Show SMILES NC(=N)NCCCC(NC(=O)CN1CCN(CC1=O)S(=O)(=O)c1ccc2c(Cl)cccc2c1)C(=O)c1nccs1 Show InChI InChI=1S/C25H28ClN7O5S2/c26-19-4-1-3-16-13-17(6-7-18(16)19)40(37,38)33-11-10-32(22(35)15-33)14-21(34)31-20(5-2-8-30-25(27)28)23(36)24-29-9-12-39-24/h1,3-4,6-7,9,12-13,20H,2,5,8,10-11,14-15H2,(H,31,34)(H4,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124992
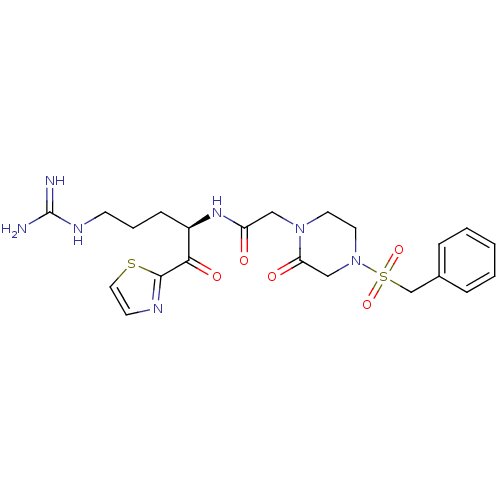
(CHEMBL162461 | N-[(R)-4-Guanidino-1-(thiazole-2-ca...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CN1CCN(CC1=O)S(=O)(=O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C22H29N7O5S2/c23-22(24)26-8-4-7-17(20(32)21-25-9-12-35-21)27-18(30)13-28-10-11-29(14-19(28)31)36(33,34)15-16-5-2-1-3-6-16/h1-3,5-6,9,12,17H,4,7-8,10-11,13-15H2,(H,27,30)(H4,23,24,26)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507688
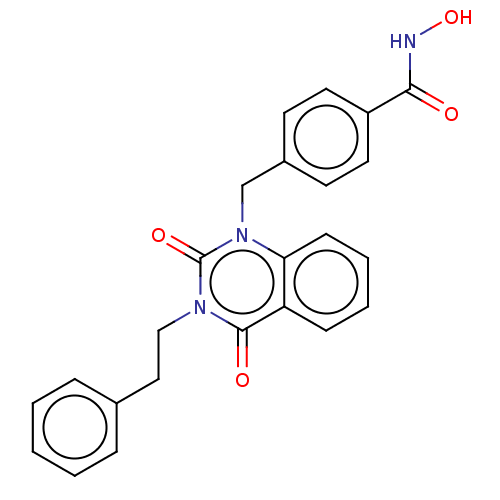
(CHEMBL4550214 | US11535598, Compound 5)Show SMILES ONC(=O)c1ccc(Cn2c3ccccc3c(=O)n(CCc3ccccc3)c2=O)cc1 Show InChI InChI=1S/C24H21N3O4/c28-22(25-31)19-12-10-18(11-13-19)16-27-21-9-5-4-8-20(21)23(29)26(24(27)30)15-14-17-6-2-1-3-7-17/h1-13,31H,14-16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate ... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047
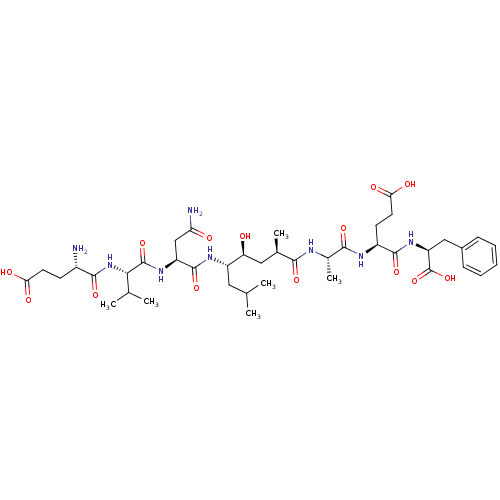
((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human beta-secretase |
Bioorg Med Chem Lett 13: 4335-9 (2003)
BindingDB Entry DOI: 10.7270/Q2513ZR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50244316
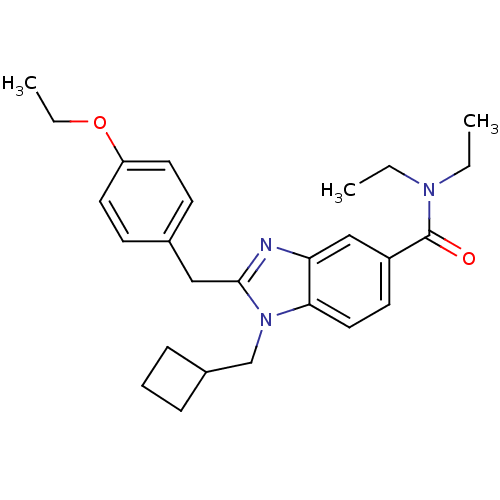
(2-(4-ethoxybenzyl)-1-(cyclobutylmethyl)-N,N-diethy...)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCC2)C(=O)N(CC)CC)cc1 Show InChI InChI=1S/C26H33N3O2/c1-4-28(5-2)26(30)21-12-15-24-23(17-21)27-25(29(24)18-20-8-7-9-20)16-19-10-13-22(14-11-19)31-6-3/h10-15,17,20H,4-9,16,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3695-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.073
BindingDB Entry DOI: 10.7270/Q2J38SBN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150413
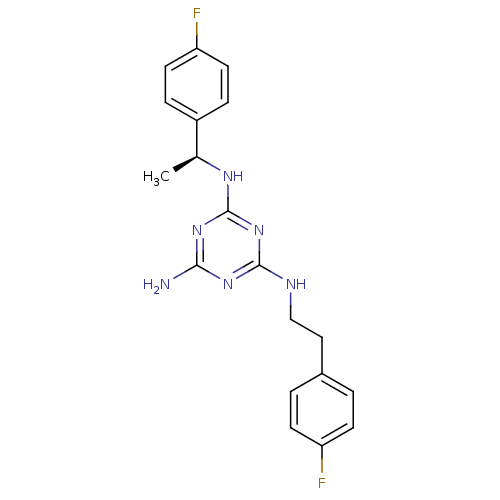
(CHEMBL413049 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-[...)Show SMILES C[C@H](Nc1nc(N)nc(NCCc2ccc(F)cc2)n1)c1ccc(F)cc1 Show InChI InChI=1S/C19H20F2N6/c1-12(14-4-8-16(21)9-5-14)24-19-26-17(22)25-18(27-19)23-11-10-13-2-6-15(20)7-3-13/h2-9,12H,10-11H2,1H3,(H4,22,23,24,25,26,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [75-147]
(Homo sapiens (Human)) | BDBM404301

(US10017501, Compound 1020-289)Show SMILES Cc1noc(C)c1-c1cc(c2nc([nH]c2c1)C1CC1)C(O)(C1CCCO1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc
US Patent
| Assay Description
Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... |
US Patent US10017501 (2018)
BindingDB Entry DOI: 10.7270/Q2CV4M3Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50596723

(CHEMBL5205903 | US20230348421, Compound 59)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cn1)C(=O)NCc1ccc(OCC(F)(F)F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114246
BindingDB Entry DOI: 10.7270/Q20P142F |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [368-440]
(Homo sapiens (Human)) | BDBM404301

(US10017501, Compound 1020-289)Show SMILES Cc1noc(C)c1-c1cc(c2nc([nH]c2c1)C1CC1)C(O)(C1CCCO1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc
US Patent
| Assay Description
Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... |
US Patent US10017501 (2018)
BindingDB Entry DOI: 10.7270/Q2CV4M3Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150407
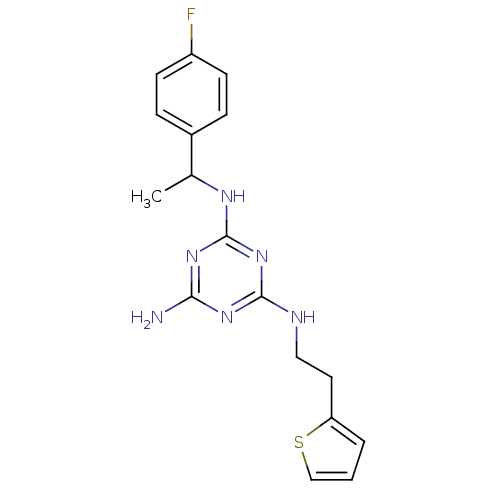
(CHEMBL180086 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...)Show InChI InChI=1S/C17H19FN6S/c1-11(12-4-6-13(18)7-5-12)21-17-23-15(19)22-16(24-17)20-9-8-14-3-2-10-25-14/h2-7,10-11H,8-9H2,1H3,(H4,19,20,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150408
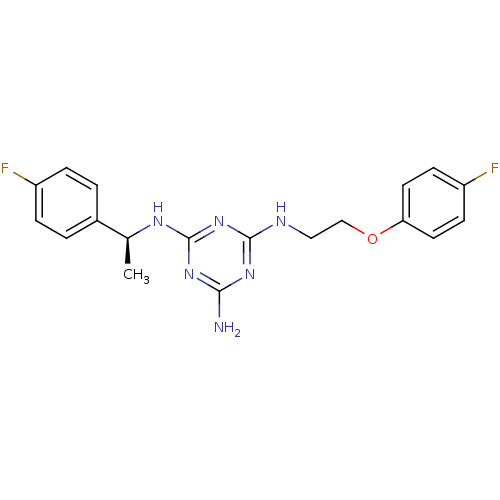
(CHEMBL182937 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...)Show SMILES C[C@H](Nc1nc(N)nc(NCCOc2ccc(F)cc2)n1)c1ccc(F)cc1 Show InChI InChI=1S/C19H20F2N6O/c1-12(13-2-4-14(20)5-3-13)24-19-26-17(22)25-18(27-19)23-10-11-28-16-8-6-15(21)7-9-16/h2-9,12H,10-11H2,1H3,(H4,22,23,24,25,26,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507695
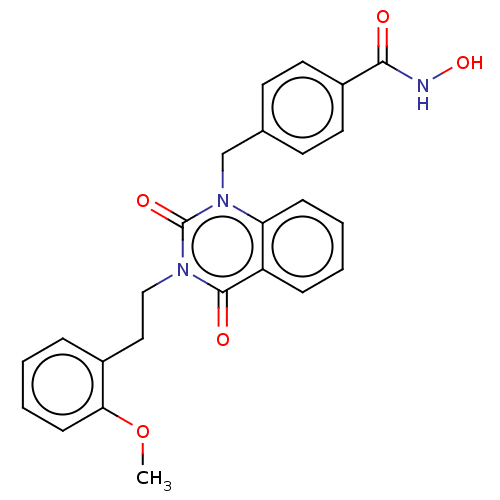
(CHEMBL4554270 | US11535598, Compound 15)Show SMILES COc1ccccc1CCn1c(=O)n(Cc2ccc(cc2)C(=O)NO)c2ccccc2c1=O Show InChI InChI=1S/C25H23N3O5/c1-33-22-9-5-2-6-18(22)14-15-27-24(30)20-7-3-4-8-21(20)28(25(27)31)16-17-10-12-19(13-11-17)23(29)26-32/h2-13,32H,14-16H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124995
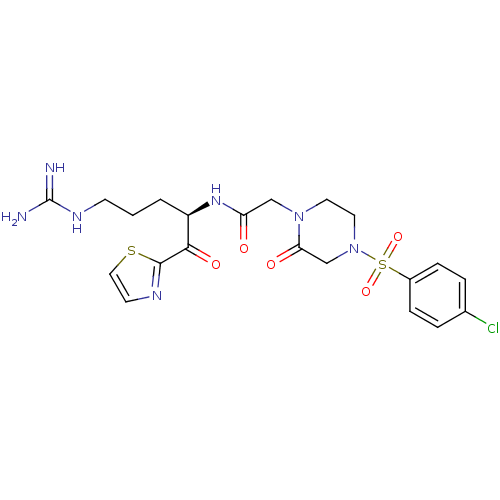
(2-[4-(4-Chloro-benzenesulfonyl)-2-oxo-piperazin-1-...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)CN1CCN(CC1=O)S(=O)(=O)c1ccc(Cl)cc1)C(=O)c1nccs1 Show InChI InChI=1S/C21H26ClN7O5S2/c22-14-3-5-15(6-4-14)36(33,34)29-10-9-28(18(31)13-29)12-17(30)27-16(2-1-7-26-21(23)24)19(32)20-25-8-11-35-20/h3-6,8,11,16H,1-2,7,9-10,12-13H2,(H,27,30)(H4,23,24,26)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50124947

(CHEMBL453539)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of bovine beta-trypsin using BAPNA as substrate preincubated for 15 mins followed by substrate addition measured over 60 mins by Dixon plo... |
J Med Chem 60: 504-510 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01011
BindingDB Entry DOI: 10.7270/Q2RJ4MRS |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16250
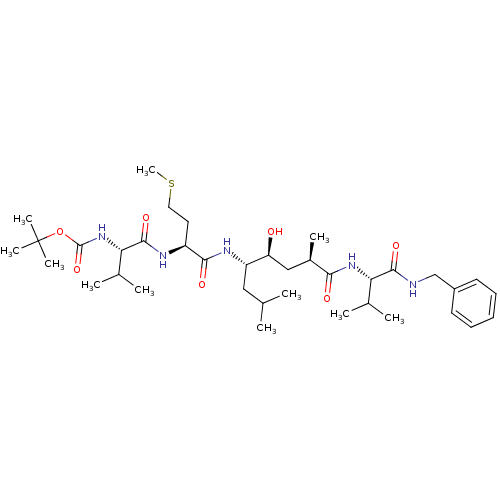
(CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C37H63N5O7S/c1-22(2)19-28(29(43)20-25(7)32(44)41-30(23(3)4)34(46)38-21-26-15-13-12-14-16-26)40-33(45)27(17-18-50-11)39-35(47)31(24(5)6)42-36(48)49-37(8,9)10/h12-16,22-25,27-31,43H,17-21H2,1-11H3,(H,38,46)(H,39,47)(H,40,45)(H,41,44)(H,42,48)/t25-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Beta-secretase-1 in HEK293 (Human Embryonic Kidney) cell line. |
Bioorg Med Chem Lett 14: 239-43 (2003)
BindingDB Entry DOI: 10.7270/Q2MW2HP3 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50387561
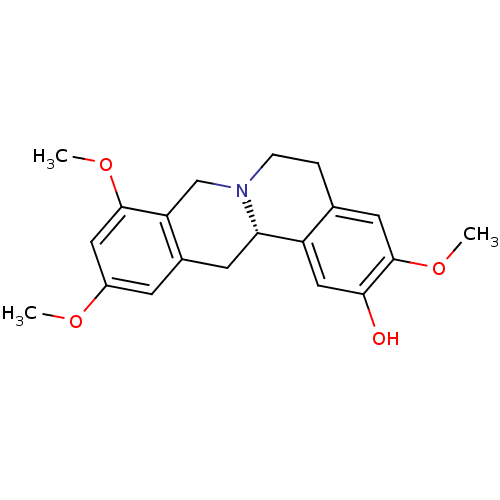
(CHEMBL2057455 | US9359372, DC037079)Show SMILES COc1cc2C[C@@H]3N(CCc4cc(OC)c(O)cc34)Cc2c(OC)c1 |r| Show InChI InChI=1S/C20H23NO4/c1-23-14-6-13-7-17-15-10-18(22)20(25-3)8-12(15)4-5-21(17)11-16(13)19(9-14)24-2/h6,8-10,17,22H,4-5,7,11H2,1-3H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter |
Bioorg Med Chem 21: 856-68 (2013)
Article DOI: 10.1016/j.bmc.2012.12.016
BindingDB Entry DOI: 10.7270/Q2JD4Z4M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507695

(CHEMBL4554270 | US11535598, Compound 15)Show SMILES COc1ccccc1CCn1c(=O)n(Cc2ccc(cc2)C(=O)NO)c2ccccc2c1=O Show InChI InChI=1S/C25H23N3O5/c1-33-22-9-5-2-6-18(22)14-15-27-24(30)20-7-3-4-8-21(20)28(25(27)31)16-17-10-12-19(13-11-17)23(29)26-32/h2-13,32H,14-16H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 CD2 expressed in Escherichia coli BL21 (RIL) using Boc-Lys (Ac)-AMC as substrate preincubated for 10 mins followed by subst... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50159089
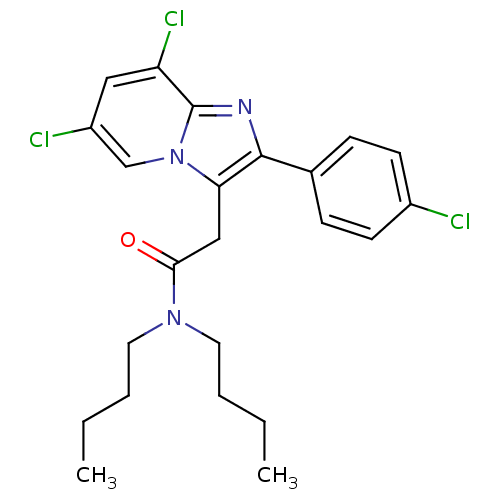
(CHEMBL180523 | N,N-Dibutyl-2-[6,8-dichloro-2-(4-ch...)Show SMILES CCCCN(CCCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H26Cl3N3O/c1-3-5-11-28(12-6-4-2)21(30)14-20-22(16-7-9-17(24)10-8-16)27-23-19(26)13-18(25)15-29(20)23/h7-10,13,15H,3-6,11-12,14H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 125: 1172-1192 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.017
BindingDB Entry DOI: 10.7270/Q25Q4Z83 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50124972
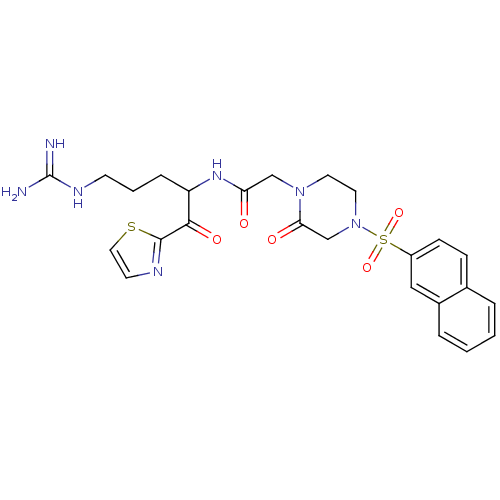
(CHEMBL163174 | N-[4-Guanidino-1-(thiazole-2-carbon...)Show SMILES NC(=N)NCCCC(NC(=O)CN1CCN(CC1=O)S(=O)(=O)c1ccc2ccccc2c1)C(=O)c1nccs1 Show InChI InChI=1S/C25H29N7O5S2/c26-25(27)29-9-3-6-20(23(35)24-28-10-13-38-24)30-21(33)15-31-11-12-32(16-22(31)34)39(36,37)19-8-7-17-4-1-2-5-18(17)14-19/h1-2,4-5,7-8,10,13-14,20H,3,6,9,11-12,15-16H2,(H,30,33)(H4,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards factor Xa |
Bioorg Med Chem Lett 13: 723-8 (2003)
BindingDB Entry DOI: 10.7270/Q2Z037JH |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [368-440]
(Homo sapiens (Human)) | BDBM249309
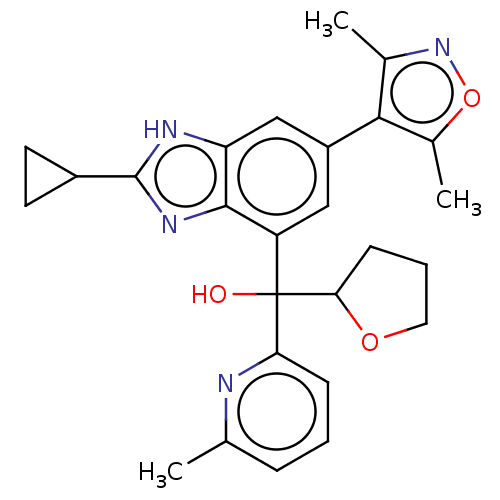
(US10017501, Compound 1020-114 | US9458145, 1020-11...)Show SMILES Cc1noc(C)c1-c1cc(c2nc([nH]c2c1)C1CC1)C(O)(C1CCCO1)c1cccc(C)n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc
US Patent
| Assay Description
Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... |
US Patent US10017501 (2018)
BindingDB Entry DOI: 10.7270/Q2CV4M3Z |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50159089

(CHEMBL180523 | N,N-Dibutyl-2-[6,8-dichloro-2-(4-ch...)Show SMILES CCCCN(CCCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H26Cl3N3O/c1-3-5-11-28(12-6-4-2)21(30)14-20-22(16-7-9-17(24)10-8-16)27-23-19(26)13-18(25)15-29(20)23/h7-10,13,15H,3-6,11-12,14H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 125: 1172-1192 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.017
BindingDB Entry DOI: 10.7270/Q25Q4Z83 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50244319
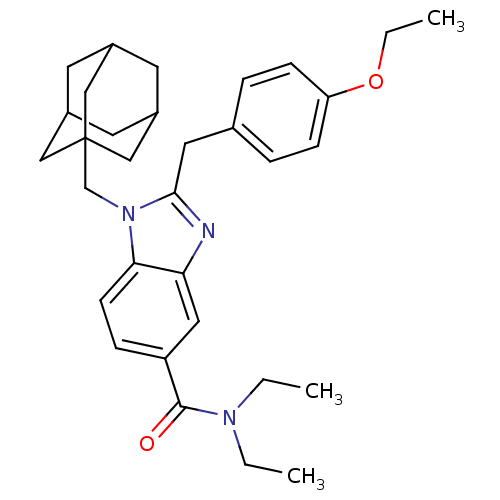
(1-adamantan-1-ylmethyl-2-(4-ethoxy-benzyl)-1H-benz...)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC23CC4CC(CC(C4)C2)C3)C(=O)N(CC)CC)cc1 |THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18| Show InChI InChI=1S/C32H41N3O2/c1-4-34(5-2)31(36)26-9-12-29-28(17-26)33-30(16-22-7-10-27(11-8-22)37-6-3)35(29)21-32-18-23-13-24(19-32)15-25(14-23)20-32/h7-12,17,23-25H,4-6,13-16,18-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3695-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.073
BindingDB Entry DOI: 10.7270/Q2J38SBN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50244060
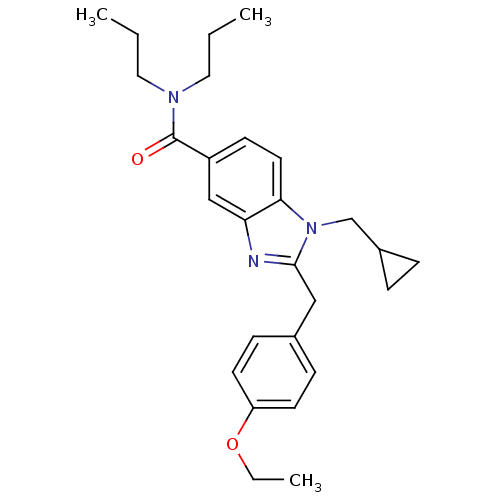
(2-(4-ethoxybenzyl)-1-(cyclopropylmethyl)-N,N-dipro...)Show SMILES CCCN(CCC)C(=O)c1ccc2n(CC3CC3)c(Cc3ccc(OCC)cc3)nc2c1 Show InChI InChI=1S/C27H35N3O2/c1-4-15-29(16-5-2)27(31)22-11-14-25-24(18-22)28-26(30(25)19-21-7-8-21)17-20-9-12-23(13-10-20)32-6-3/h9-14,18,21H,4-8,15-17,19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor |
Bioorg Med Chem Lett 18: 3695-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.073
BindingDB Entry DOI: 10.7270/Q2J38SBN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
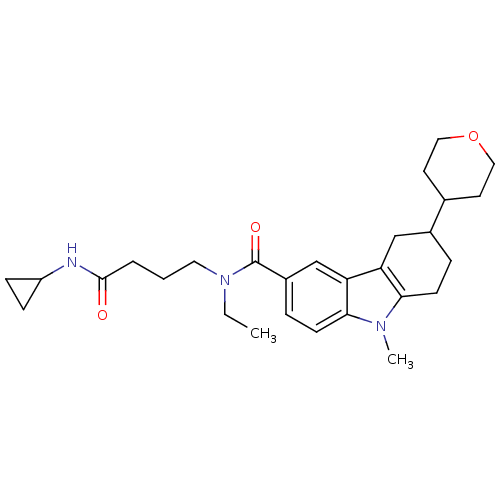
(Homo sapiens (Human)) | BDBM50384144
(CHEMBL2029719)Show SMILES CCN(CCCC(=O)NC1CC1)C(=O)c1ccc2n(C)c3CCC(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C28H39N3O3/c1-3-31(14-4-5-27(32)29-22-8-9-22)28(33)21-7-11-26-24(18-21)23-17-20(6-10-25(23)30(26)2)19-12-15-34-16-13-19/h7,11,18-20,22H,3-6,8-10,12-17H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 17: 6183-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.019
BindingDB Entry DOI: 10.7270/Q2TH8MD3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
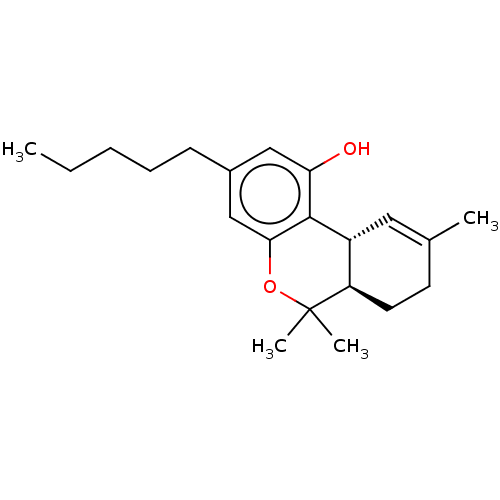
(Homo sapiens (Human)) | BDBM60994
((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor |
Bioorg Med Chem Lett 18: 3695-700 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.073
BindingDB Entry DOI: 10.7270/Q2J38SBN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ketanserin from 5-HT2A receptor (unknown origin) expressed in HEK293 cells by liquid scintillation counter |
Bioorg Med Chem 21: 856-68 (2013)
Article DOI: 10.1016/j.bmc.2012.12.016
BindingDB Entry DOI: 10.7270/Q2JD4Z4M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
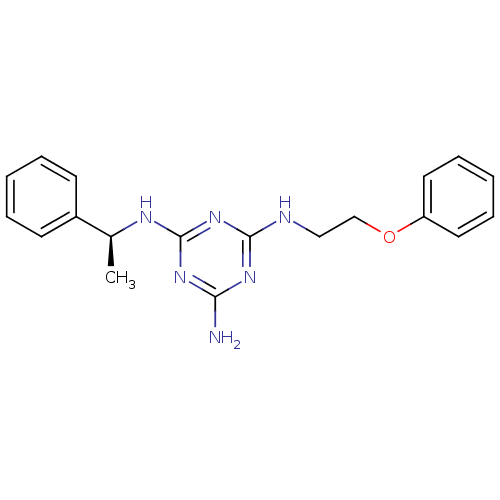
(Homo sapiens (Human)) | BDBM50150410
(CHEMBL182174 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...)Show InChI InChI=1S/C19H22N6O/c1-14(15-8-4-2-5-9-15)22-19-24-17(20)23-18(25-19)21-12-13-26-16-10-6-3-7-11-16/h2-11,14H,12-13H2,1H3,(H4,20,21,22,23,24,25)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150412
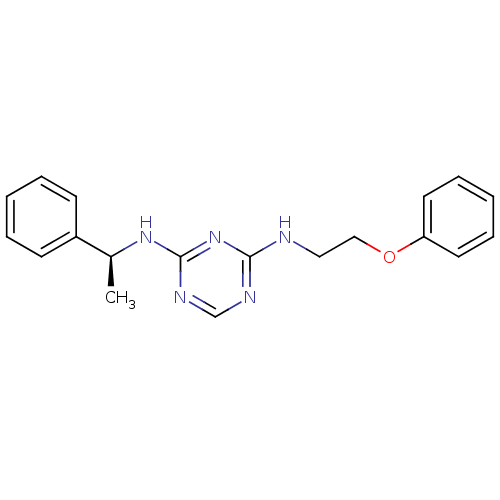
(CHEMBL183862 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...)Show InChI InChI=1S/C19H21N5O/c1-15(16-8-4-2-5-9-16)23-19-22-14-21-18(24-19)20-12-13-25-17-10-6-3-7-11-17/h2-11,14-15H,12-13H2,1H3,(H2,20,21,22,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50045877
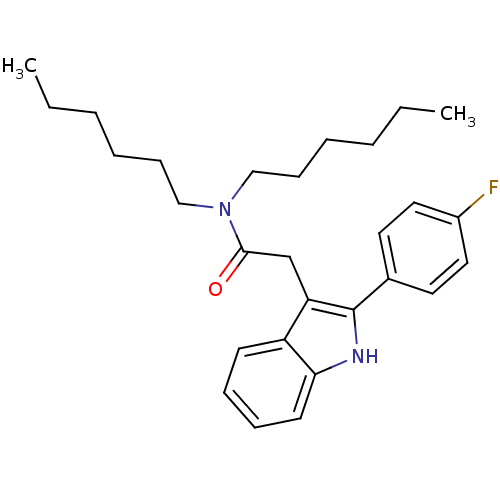
(2-(2-(4-fluorophenyl)-1H-indol-3-yl)-N,N-dihexylac...)Show SMILES CCCCCCN(CCCCCC)C(=O)Cc1c([nH]c2ccccc12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H37FN2O/c1-3-5-7-11-19-31(20-12-8-6-4-2)27(32)21-25-24-13-9-10-14-26(24)30-28(25)22-15-17-23(29)18-16-22/h9-10,13-18,30H,3-8,11-12,19-21H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 125: 1172-1192 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.017
BindingDB Entry DOI: 10.7270/Q25Q4Z83 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50045877

(2-(2-(4-fluorophenyl)-1H-indol-3-yl)-N,N-dihexylac...)Show SMILES CCCCCCN(CCCCCC)C(=O)Cc1c([nH]c2ccccc12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H37FN2O/c1-3-5-7-11-19-31(20-12-8-6-4-2)27(32)21-25-24-13-9-10-14-26(24)30-28(25)22-15-17-23(29)18-16-22/h9-10,13-18,30H,3-8,11-12,19-21H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 125: 1172-1192 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.017
BindingDB Entry DOI: 10.7270/Q25Q4Z83 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50378584
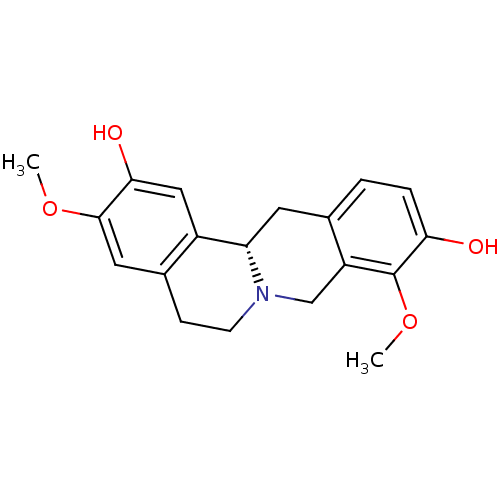
(STEPHOLIDINE)Show SMILES COc1cc2CCN3Cc4c(C[C@H]3c2cc1O)ccc(O)c4OC |r| Show InChI InChI=1S/C19H21NO4/c1-23-18-8-12-5-6-20-10-14-11(3-4-16(21)19(14)24-2)7-15(20)13(12)9-17(18)22/h3-4,8-9,15,21-22H,5-7,10H2,1-2H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in human HEK293 cells by liquid scintillation counter |
Bioorg Med Chem 21: 856-68 (2013)
Article DOI: 10.1016/j.bmc.2012.12.016
BindingDB Entry DOI: 10.7270/Q2JD4Z4M |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AnnJi Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human full-length recombinant HDAC6 expressed in baculovirus infected Sf9 insect cells using Boc-Lys (Ac)-AMC as substrate preincubated... |
J Med Chem 62: 857-874 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01590
BindingDB Entry DOI: 10.7270/Q2833WBH |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114424
BindingDB Entry DOI: 10.7270/Q2VX0MHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data