| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urokinase-type plasminogen activator |
|---|
| Ligand | BDBM50153004 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1564079 (CHEMBL3783220) |
|---|
| Ki | >29730±n/a nM |
|---|
| Citation |  Corte, JR; Fang, T; Pinto, DJ; Orwat, MJ; Rendina, AR; Luettgen, JM; Rossi, KA; Wei, A; Ramamurthy, V; Myers, JE; Sheriff, S; Narayanan, R; Harper, TW; Zheng, JJ; Li, YX; Seiffert, DA; Wexler, RR; Quan, ML Orally bioavailable pyridine and pyrimidine-based Factor XIa inhibitors: Discovery of the methyl N-phenyl carbamate P2 prime group. Bioorg Med Chem24:2257-72 (2016) [PubMed] Article Corte, JR; Fang, T; Pinto, DJ; Orwat, MJ; Rendina, AR; Luettgen, JM; Rossi, KA; Wei, A; Ramamurthy, V; Myers, JE; Sheriff, S; Narayanan, R; Harper, TW; Zheng, JJ; Li, YX; Seiffert, DA; Wexler, RR; Quan, ML Orally bioavailable pyridine and pyrimidine-based Factor XIa inhibitors: Discovery of the methyl N-phenyl carbamate P2 prime group. Bioorg Med Chem24:2257-72 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urokinase-type plasminogen activator |
|---|
| Name: | Urokinase-type plasminogen activator |
|---|
| Synonyms: | 3.4.21.73 | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator chain B | Urokinase-type plasminogen activator long chain A | Urokinase-type plasminogen activator short chain A | Urokinase-type plasminogen activator/surface receptor | uPA |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48528.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00749 |
|---|
| Residue: | 431 |
|---|
| Sequence: | MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQ
HCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHN
YCRNPDNRRRPWCYVQVGLKLLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKII
GGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLG
RSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICL
PSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKML
CAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIR
SHTKEENGLAL
|
|
|
|---|
| BDBM50153004 |
|---|
| n/a |
|---|
| Name | BDBM50153004 |
|---|
| Synonyms: | CHEMBL3781319 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H26ClN7O3 |
|---|
| Mol. Mass. | 580.036 |
|---|
| SMILES | COC(=O)Nc1ccc(cc1)-c1ccnc(c1)[C@H](Cc1ccccc1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| |
|---|
| Structure | 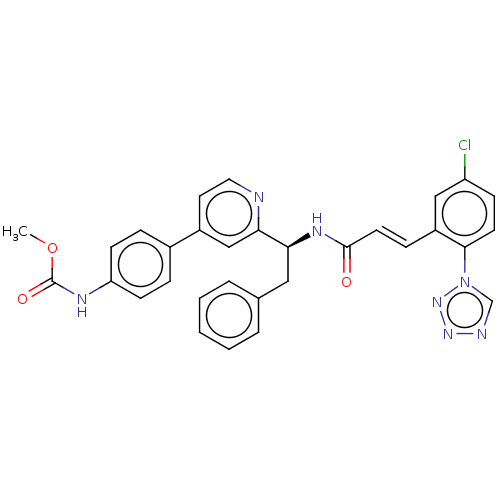
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Corte, JR; Fang, T; Pinto, DJ; Orwat, MJ; Rendina, AR; Luettgen, JM; Rossi, KA; Wei, A; Ramamurthy, V; Myers, JE; Sheriff, S; Narayanan, R; Harper, TW; Zheng, JJ; Li, YX; Seiffert, DA; Wexler, RR; Quan, ML Orally bioavailable pyridine and pyrimidine-based Factor XIa inhibitors: Discovery of the methyl N-phenyl carbamate P2 prime group. Bioorg Med Chem24:2257-72 (2016) [PubMed] Article
Corte, JR; Fang, T; Pinto, DJ; Orwat, MJ; Rendina, AR; Luettgen, JM; Rossi, KA; Wei, A; Ramamurthy, V; Myers, JE; Sheriff, S; Narayanan, R; Harper, TW; Zheng, JJ; Li, YX; Seiffert, DA; Wexler, RR; Quan, ML Orally bioavailable pyridine and pyrimidine-based Factor XIa inhibitors: Discovery of the methyl N-phenyl carbamate P2 prime group. Bioorg Med Chem24:2257-72 (2016) [PubMed] Article