 Found 12 hits for monomerid = 14320
Found 12 hits for monomerid = 14320 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14320
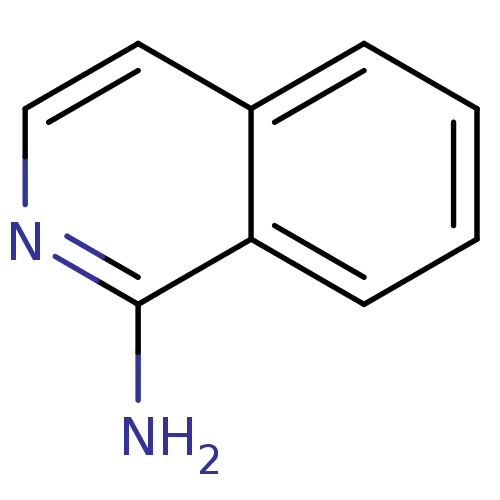
(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of urokinase |
J Med Chem 52: 3159-65 (2009)
Article DOI: 10.1021/jm801444x
BindingDB Entry DOI: 10.7270/Q2FF3TM8 |
More data for this
Ligand-Target Pair | |
Tryptase
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Neutrophil cytosol factor 1
(Homo sapiens) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Covalent inhibition of recombinant human His-tagged p47phox SH3A-B domain (151 to 285 residues) expressed in Escherichia coli BL21 (DE3) cells intera... |
J Med Chem 63: 1156-1177 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01492 |
More data for this
Ligand-Target Pair | |
Trypsin
(Bos taurus (bovine)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Biochemistry 45: 5964-73 (2006)
Article DOI: 10.1021/bi060173m
BindingDB Entry DOI: 10.7270/Q2W09450 |
More data for this
Ligand-Target Pair | |
Coagulation factor III/Factor VIIa (fVIIa)
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human factor-7a/TF using S2288 as substrate measured after 60 mins |
ACS Med Chem Lett 7: 1077-1081 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00282
BindingDB Entry DOI: 10.7270/Q2GB2613 |
More data for this
Ligand-Target Pair | |
Neutrophil cytosol factor 1
(Homo sapiens) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.87E+5 | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Binding affinity to immobilized recombinant human His-tagged p47phox SH3A-B domain (151 to 285 residues) expressed in Escherichia coli BL21 (DE3) cel... |
J Med Chem 63: 1156-1177 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01492 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TEC
(Mus musculus) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide
Curated by ChEMBL
| Assay Description
Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization |
J Med Chem 47: 5405-17 (2004)
Article DOI: 10.1021/jm049533z
BindingDB Entry DOI: 10.7270/Q26M369B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Astex
| Assay Description
Activity of BACE-1 was measured by monitoring the cleavage of its peptide substrate on a Fluoroskan Ascent plate reader with excitation and emission ... |
J Med Chem 50: 1116-23 (2007)
Article DOI: 10.1021/jm0611962
BindingDB Entry DOI: 10.7270/Q2Q23XHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM14320

(1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...)Show InChI InChI=1S/C9H8N2/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway
Curated by ChEMBL
| Assay Description
Inhibitory activity against inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 10: 1975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2319V30 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data