

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50262422 CHEMBL477339::sodium (E)-4-((3-formyl-4-hydroxy-5-methyl-2-(phosphonatooxymethyl)phenyl)diazenyl)benzene-1,3-disulfonate
SMILES: Cc1cc(N=Nc2ccc(cc2S([O-])(=O)=O)S([O-])(=O)=O)c(COP([O-])([O-])=O)c(C=O)c1O
InChI Key: InChIKey=FFLAALLXNFGXRL-UHFFFAOYSA-J
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleoside triphosphate diphosphohydrolase 2 (Rattus norvegicus) | BDBM50262422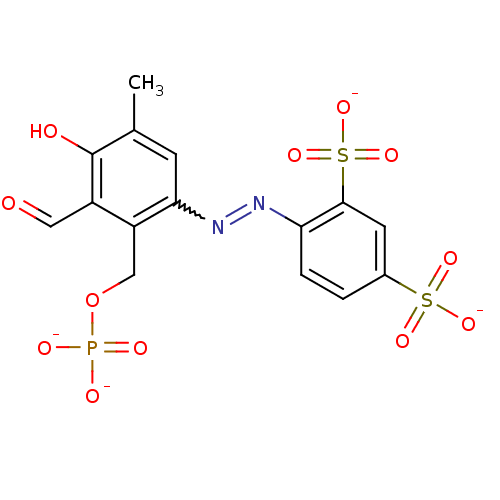 (CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat NTPDase 2 | J Med Chem 51: 4518-28 (2008) Article DOI: 10.1021/jm800175e BindingDB Entry DOI: 10.7270/Q2XW4KQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleoside triphosphate diphosphohydrolase 1 (Rattus norvegicus) | BDBM50262422 (CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat NTPDase 1 | J Med Chem 51: 4518-28 (2008) Article DOI: 10.1021/jm800175e BindingDB Entry DOI: 10.7270/Q2XW4KQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y6 (Homo sapiens (Human)) | BDBM50262422 (CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as inhibition of UDP-induced intracellular calcium mobilization b... | J Med Chem 51: 4518-28 (2008) Article DOI: 10.1021/jm800175e BindingDB Entry DOI: 10.7270/Q2XW4KQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic, P2X2 (RAT) | BDBM50262422 (CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity against rat P2X2 receptor expressed in Xenopus laevis oocyte assessed as inhibition of alpha, beta-meATP-induced inward current b... | J Med Chem 54: 817-30 (2012) Article DOI: 10.1021/jm1012193 BindingDB Entry DOI: 10.7270/Q2VH5PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y4 (Homo sapiens (Human)) | BDBM50262422 (CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2Y4 receptor expressed in human 1321N1 cells assessed as inhibition of UTP-induced intracellular calcium mobilization b... | J Med Chem 51: 4518-28 (2008) Article DOI: 10.1021/jm800175e BindingDB Entry DOI: 10.7270/Q2XW4KQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||