 Found 83 hits Enz. Inhib. hit(s) with Target = 'Ectonucleoside triphosphate diphosphohydrolase 1'
Found 83 hits Enz. Inhib. hit(s) with Target = 'Ectonucleoside triphosphate diphosphohydrolase 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50378649
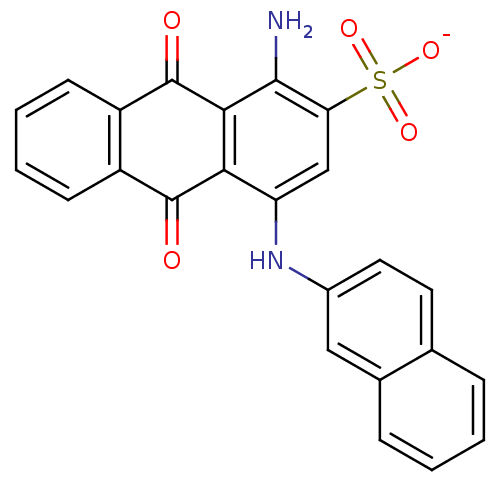
(CHEMBL597197)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2ccc3ccccc3c2)cc1S([O-])(=O)=O Show InChI InChI=1S/C24H16N2O5S/c25-22-19(32(29,30)31)12-18(26-15-10-9-13-5-1-2-6-14(13)11-15)20-21(22)24(28)17-8-4-3-7-16(17)23(20)27/h1-12,26H,25H2,(H,29,30,31)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat CD39 expressed in CHO cells using ATP as substrate incubated for 10 mins by UV absorbance method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50010311
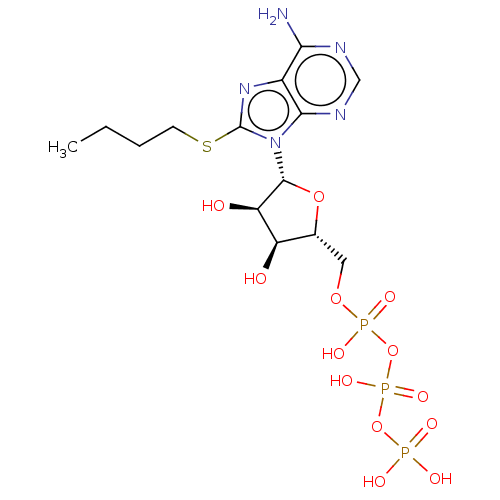
(CHEMBL2364580)Show SMILES CCCCSc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H24N5O13P3S/c1-2-3-4-36-14-18-8-11(15)16-6-17-12(8)19(14)13-10(21)9(20)7(30-13)5-29-34(25,26)32-35(27,28)31-33(22,23)24/h6-7,9-10,13,20-21H,2-5H2,1H3,(H,25,26)(H,27,28)(H2,15,16,17)(H2,22,23,24)/t7-,9-,10-,13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 using ATP as substrate preincubated for 3 mins followed by substrate addition and measured after 10 to 15 mins by Cheng-Prus... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50552127
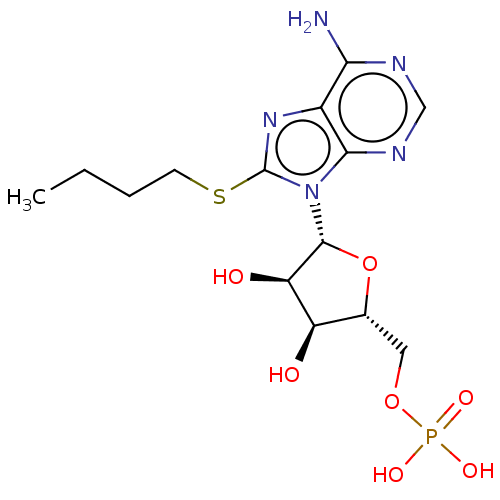
(CHEMBL4750781)Show SMILES CCCCSc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 using ATP as substrate preincubated for 3 mins followed by substrate addition and measured after 10 to 15 mins by Dixon and ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50552126
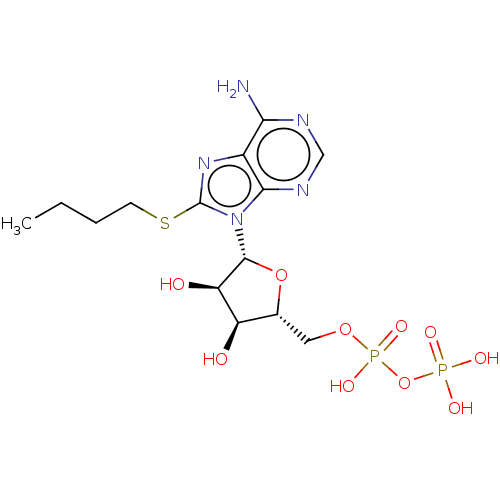
(CHEMBL4797422)Show SMILES CCCCSc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 using ATP as substrate preincubated for 3 mins followed by substrate addition and measured after 10 to 15 mins by Dixon and ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50307836
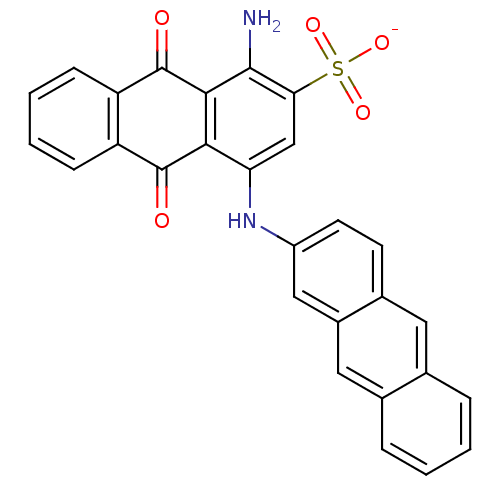
(CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2ccc3cc4ccccc4cc3c2)cc1S([O-])(=O)=O Show InChI InChI=1S/C28H18N2O5S/c29-26-23(36(33,34)35)14-22(24-25(26)28(32)21-8-4-3-7-20(21)27(24)31)30-19-10-9-17-11-15-5-1-2-6-16(15)12-18(17)13-19/h1-14,30H,29H2,(H,33,34,35)/p-1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Bos taurus) | BDBM50369522
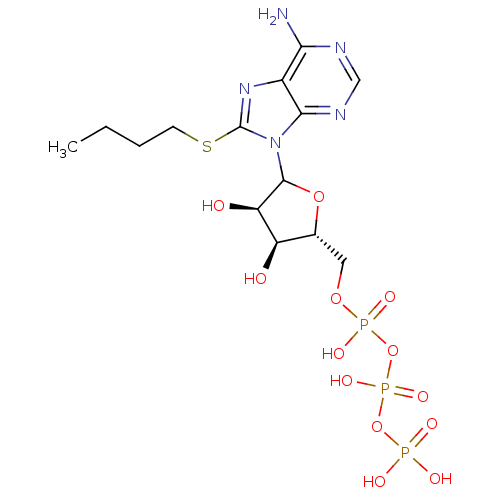
(CHEMBL610358)Show SMILES CCCCSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H24N5O13P3S/c1-2-3-4-36-14-18-8-11(15)16-6-17-12(8)19(14)13-10(21)9(20)7(30-13)5-29-34(25,26)32-35(27,28)31-33(22,23)24/h6-7,9-10,13,20-21H,2-5H2,1H3,(H,25,26)(H,27,28)(H2,15,16,17)(H2,22,23,24)/t7-,9-,10-,13?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Tested for inhibition of Nucleoside Triphosphate Diphosphohydrolase (NTPDase) from bovine spleen. |
J Med Chem 43: 2239-47 (2000)
BindingDB Entry DOI: 10.7270/Q2V40VX6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50268725
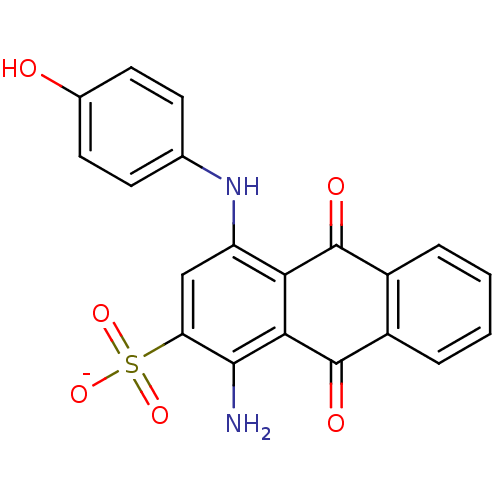
(CHEMBL496030 | sodium 1-amino-4-(4-hydroxyphenylam...)Show SMILES Nc1c(cc(Nc2ccc(O)cc2)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C20H14N2O6S/c21-18-15(29(26,27)28)9-14(22-10-5-7-11(23)8-6-10)16-17(18)20(25)13-4-2-1-3-12(13)19(16)24/h1-9,22-23H,21H2,(H,26,27,28)/p-1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50179358

(CHEMBL1206141)Show SMILES CCN(CC)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)C(Br)(Br)P(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C15H24Br2N5O12P3/c1-3-21(4-2)12-9-13(19-6-18-12)22(7-20-9)14-11(24)10(23)8(33-14)5-32-37(30,31)34-36(28,29)15(16,17)35(25,26)27/h6-8,10-11,14,23-24H,3-5H2,1-2H3,(H,28,29)(H,30,31)(H2,25,26,27)/t8-,10-,11-,14-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human CD39 using ATP as substrate preincubated for 3 mins followed by substrate addition and measured after 15 mins by Dixo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50227019
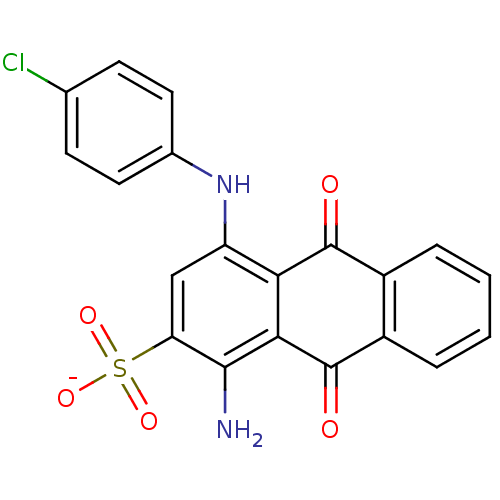
(CHEMBL271673 | sodium 1-amino-4-(4-chlorophenylami...)Show SMILES Nc1c(cc(Nc2ccc(Cl)cc2)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C20H13ClN2O5S/c21-10-5-7-11(8-6-10)23-14-9-15(29(26,27)28)18(22)17-16(14)19(24)12-3-1-2-4-13(12)20(17)25/h1-9,23H,22H2,(H,26,27,28)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat CD39 expressed in CHO cells using ATP as substrate incubated for 10 mins by UV absorbance method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Bos taurus) | BDBM50369524
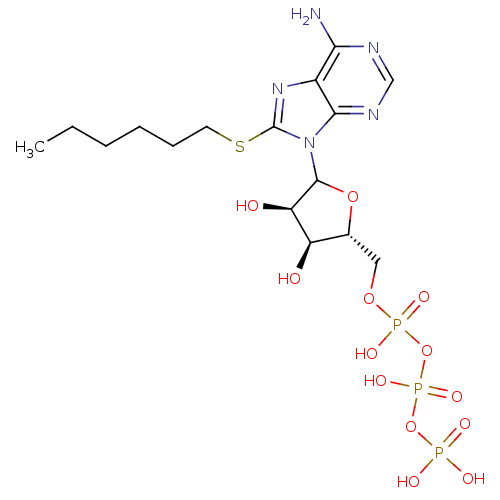
(CHEMBL607720)Show SMILES CCCCCCSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H28N5O13P3S/c1-2-3-4-5-6-38-16-20-10-13(17)18-8-19-14(10)21(16)15-12(23)11(22)9(32-15)7-31-36(27,28)34-37(29,30)33-35(24,25)26/h8-9,11-12,15,22-23H,2-7H2,1H3,(H,27,28)(H,29,30)(H2,17,18,19)(H2,24,25,26)/t9-,11-,12-,15?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Tested for inhibition of Nucleoside Triphosphate Diphosphohydrolase (NTPDase) from bovine spleen. |
J Med Chem 43: 2239-47 (2000)
BindingDB Entry DOI: 10.7270/Q2V40VX6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50029031
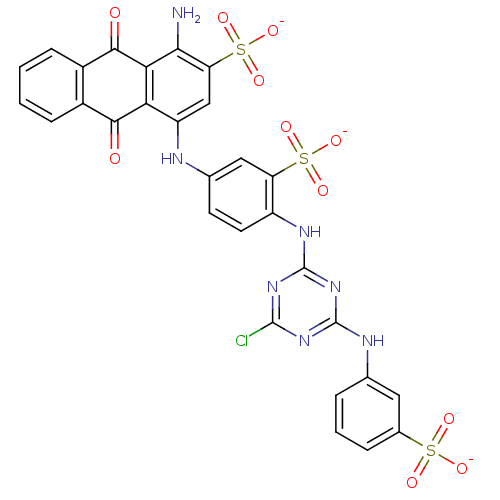
(1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)/p-3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50552129
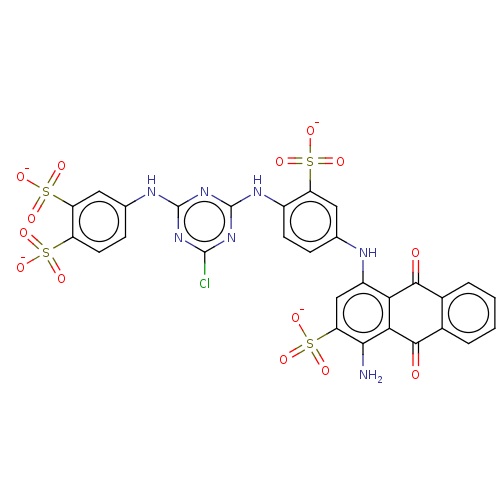
(CHEMBL4744335)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1c(cc(-[#7]-c2ccc(-[#7]-c3nc(Cl)nc(-[#7]-c4ccc(c(c4)S([#8-])(=O)=O)S([#8-])(=O)=O)n3)c(c2)S([#8-])(=O)=O)c2-[#6](=O)-c3ccccc3-[#6](=O)-c12)S([#8-])(=O)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat CD39 expressed in CHO cells using ATP as substrate incubated for 10 mins by UV absorbance method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50029031

(1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)/p-3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant NTPDase1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5943-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.003
BindingDB Entry DOI: 10.7270/Q27H1KC9 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50029031

(1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)/p-3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50195359
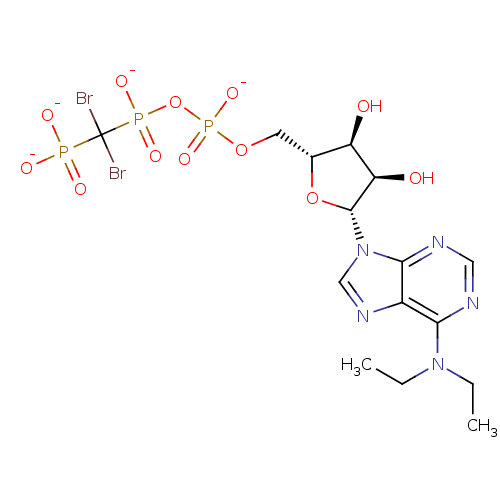
(ARL-67156 | CHEMBL223145)Show SMILES CCN(CC)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)C(Br)(Br)P([O-])([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H24Br2N5O12P3/c1-3-21(4-2)12-9-13(19-6-18-12)22(7-20-9)14-11(24)10(23)8(33-14)5-32-37(30,31)34-36(28,29)15(16,17)35(25,26)27/h6-8,10-11,14,23-24H,3-5H2,1-2H3,(H,28,29)(H,30,31)(H2,25,26,27)/p-4/t8-,10-,11-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50195359

(ARL-67156 | CHEMBL223145)Show SMILES CCN(CC)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)C(Br)(Br)P([O-])([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H24Br2N5O12P3/c1-3-21(4-2)12-9-13(19-6-18-12)22(7-20-9)14-11(24)10(23)8(33-14)5-32-37(30,31)34-36(28,29)15(16,17)35(25,26)27/h6-8,10-11,14,23-24H,3-5H2,1-2H3,(H,28,29)(H,30,31)(H2,25,26,27)/p-4/t8-,10-,11-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant NTPDase1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5943-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.003
BindingDB Entry DOI: 10.7270/Q27H1KC9 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Bos taurus) | BDBM50369523
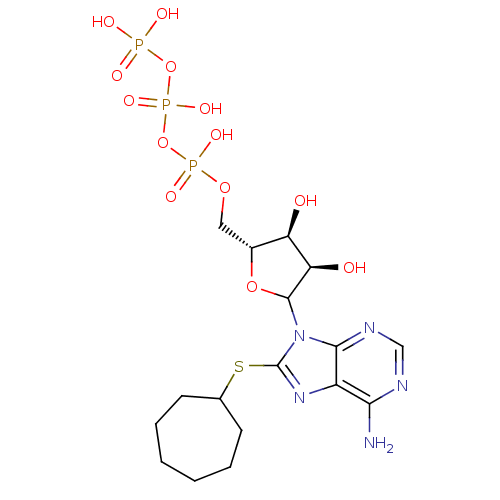
(CHEMBL612159)Show SMILES Nc1ncnc2n(C3O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]3O)c(SC3CCCCCC3)nc12 |r| Show InChI InChI=1S/C17H28N5O13P3S/c18-14-11-15(20-8-19-14)22(17(21-11)39-9-5-3-1-2-4-6-9)16-13(24)12(23)10(33-16)7-32-37(28,29)35-38(30,31)34-36(25,26)27/h8-10,12-13,16,23-24H,1-7H2,(H,28,29)(H,30,31)(H2,18,19,20)(H2,25,26,27)/t10-,12-,13-,16?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Tested for inhibition of Nucleoside Triphosphate Diphosphohydrolase (NTPDase) from bovine spleen. |
J Med Chem 43: 2239-47 (2000)
BindingDB Entry DOI: 10.7270/Q2V40VX6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50307835
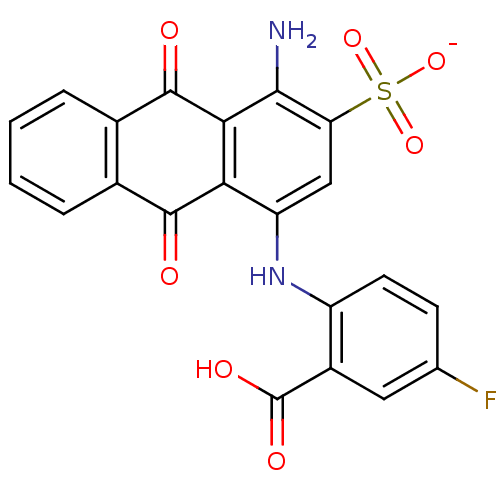
(CHEMBL590956 | Sodium 1-amino-4-[4-fluoro-2-carbox...)Show SMILES Nc1c(cc(Nc2ccc(F)cc2C(O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C21H13FN2O7S/c22-9-5-6-13(12(7-9)21(27)28)24-14-8-15(32(29,30)31)18(23)17-16(14)19(25)10-3-1-2-4-11(10)20(17)26/h1-8,24H,23H2,(H,27,28)(H,29,30,31)/p-1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Bos taurus) | BDBM50369521
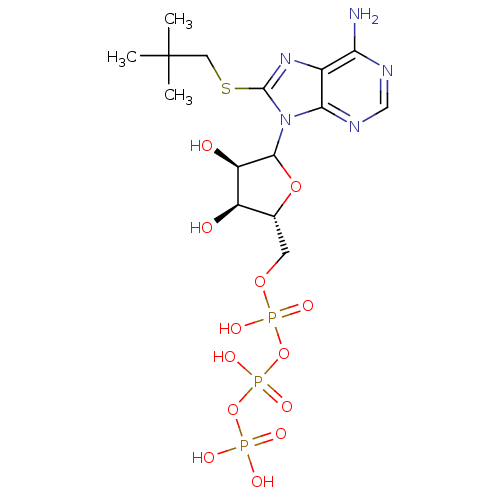
(CHEMBL610939)Show SMILES CC(C)(C)CSc1nc2c(N)ncnc2n1C1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H26N5O13P3S/c1-15(2,3)5-37-14-19-8-11(16)17-6-18-12(8)20(14)13-10(22)9(21)7(31-13)4-30-35(26,27)33-36(28,29)32-34(23,24)25/h6-7,9-10,13,21-22H,4-5H2,1-3H3,(H,26,27)(H,28,29)(H2,16,17,18)(H2,23,24,25)/t7-,9-,10-,13?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke
Curated by ChEMBL
| Assay Description
Tested for inhibition of Nucleoside Triphosphate Diphosphohydrolase (NTPDase) from bovine spleen. |
J Med Chem 43: 2239-47 (2000)
BindingDB Entry DOI: 10.7270/Q2V40VX6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50262422
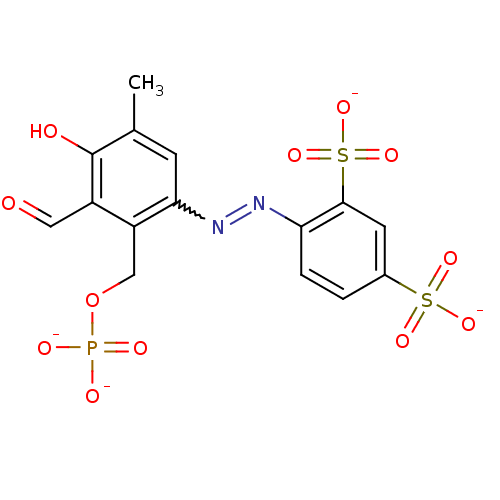
(CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...)Show SMILES Cc1cc(N=Nc2ccc(cc2S([O-])(=O)=O)S([O-])(=O)=O)c(COP([O-])([O-])=O)c(C=O)c1O |w:4.3| Show InChI InChI=1S/C15H15N2O12PS2/c1-8-4-13(11(7-29-30(20,21)22)10(6-18)15(8)19)17-16-12-3-2-9(31(23,24)25)5-14(12)32(26,27)28/h2-6,19H,7H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)/p-4 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50227032
(CHEMBL256057 | acid blue 25 | sodium 1-amino-9,10-...)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2ccccc2)cc1S([O-])(=O)=O Show InChI InChI=1S/C20H14N2O5S/c21-18-15(28(25,26)27)10-14(22-11-6-2-1-3-7-11)16-17(18)20(24)13-9-5-4-8-12(13)19(16)23/h1-10,22H,21H2,(H,25,26,27)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50262250
(4-[3-((2S,3R,4S,5R)-5-(2,4-Dioxo-3,4-dihydropyrimi...)Show SMILES CCOP(=O)(Cc1ccc(NC(=O)CCNC(=O)[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)cc1)OCC |r| Show InChI InChI=1S/C23H31N4O10P/c1-3-35-38(34,36-4-2)13-14-5-7-15(8-6-14)25-16(28)9-11-24-21(32)20-18(30)19(31)22(37-20)27-12-10-17(29)26-23(27)33/h5-8,10,12,18-20,22,30-31H,3-4,9,11,13H2,1-2H3,(H,24,32)(H,25,28)(H,26,29,33)/t18-,19+,20-,22+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPDase 1 expressed in COS7 cells |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50336767
(CHEMBL257495 | PSB-716 | sodium 1-amino-4-(2-metho...)Show SMILES COc1ccccc1Nc1cc(c(N)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C21H16N2O6S/c1-29-15-9-5-4-8-13(15)23-14-10-16(30(26,27)28)19(22)18-17(14)20(24)11-6-2-3-7-12(11)21(18)25/h2-10,23H,22H2,1H3,(H,26,27,28)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
Bioorg Med Chem Lett 18: 223-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.082
BindingDB Entry DOI: 10.7270/Q2RX9CXT |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50268574
(CHEMBL498423 | Sodium 1-Amino-4-(1-naphthylamino)-...)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2cccc3ccccc23)cc1S([O-])(=O)=O Show InChI InChI=1S/C24H16N2O5S/c25-22-19(32(29,30)31)12-18(26-17-11-5-7-13-6-1-2-8-14(13)17)20-21(22)24(28)16-10-4-3-9-15(16)23(20)27/h1-12,26H,25H2,(H,29,30,31)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50262372
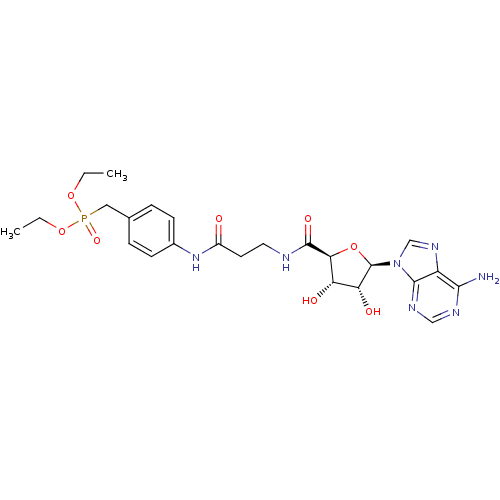
(4-[3-((2S,3R,4S,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-...)Show SMILES CCOP(=O)(Cc1ccc(NC(=O)CCNC(=O)[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)cc1)OCC |r| Show InChI InChI=1S/C24H32N7O8P/c1-3-37-40(36,38-4-2)11-14-5-7-15(8-6-14)30-16(32)9-10-26-23(35)20-18(33)19(34)24(39-20)31-13-29-17-21(25)27-12-28-22(17)31/h5-8,12-13,18-20,24,33-34H,3-4,9-11H2,1-2H3,(H,26,35)(H,30,32)(H2,25,27,28)/t18-,19+,20-,24+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPDase 1 expressed in COS7 cells |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50262373
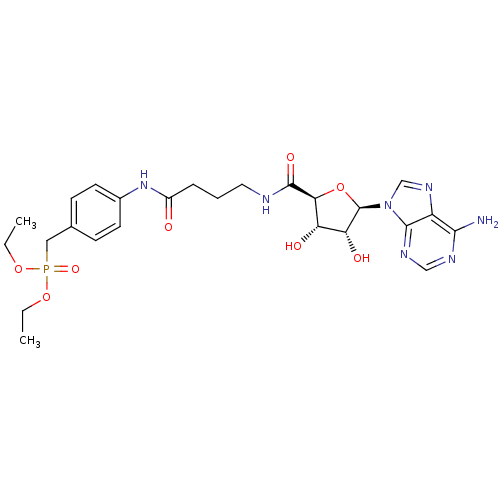
(4-[4-((2S,3R,4S,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-...)Show SMILES CCOP(=O)(Cc1ccc(NC(=O)CCCNC(=O)[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)cc1)OCC |r| Show InChI InChI=1S/C25H34N7O8P/c1-3-38-41(37,39-4-2)12-15-7-9-16(10-8-15)31-17(33)6-5-11-27-24(36)21-19(34)20(35)25(40-21)32-14-30-18-22(26)28-13-29-23(18)32/h7-10,13-14,19-21,25,34-35H,3-6,11-12H2,1-2H3,(H,27,36)(H,31,33)(H2,26,28,29)/t19-,20+,21-,25+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPDase 1 expressed in COS7 cells |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50262251
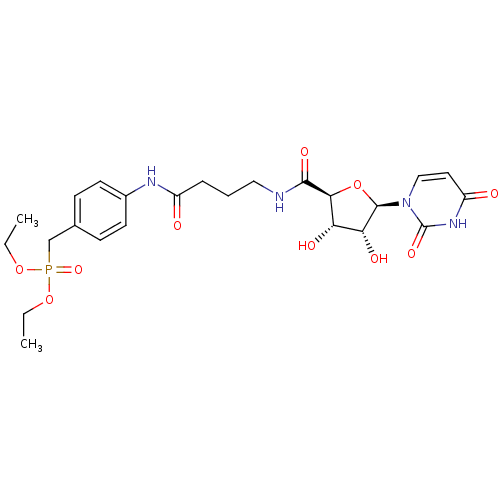
(4-[4-((2S,3R,4S,5R)-5-(2,4-Dioxo-3,4-dihydropyrimi...)Show SMILES CCOP(=O)(Cc1ccc(NC(=O)CCCNC(=O)[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)cc1)OCC |r| Show InChI InChI=1S/C24H33N4O10P/c1-3-36-39(35,37-4-2)14-15-7-9-16(10-8-15)26-17(29)6-5-12-25-22(33)21-19(31)20(32)23(38-21)28-13-11-18(30)27-24(28)34/h7-11,13,19-21,23,31-32H,3-6,12,14H2,1-2H3,(H,25,33)(H,26,29)(H,27,30,34)/t19-,20+,21-,23+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPDase 1 expressed in COS7 cells |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50336799
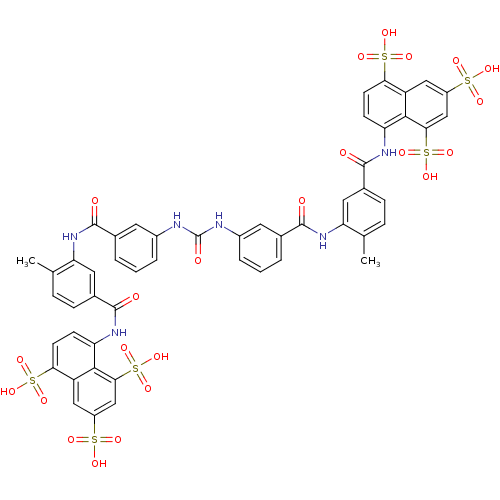
(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50336799

(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant NTPDase1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5943-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.003
BindingDB Entry DOI: 10.7270/Q27H1KC9 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50262252
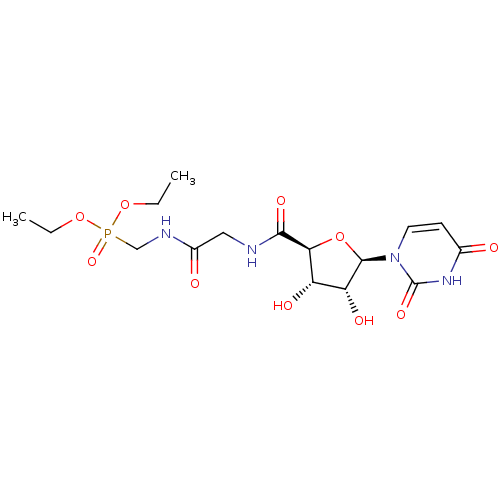
(2-[(2S,3R,4S,5R)-5-(2,4-Dioxo-3,4-dihydropyrimidin...)Show SMILES CCOP(=O)(CNC(=O)CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)OCC |r| Show InChI InChI=1S/C16H25N4O10P/c1-3-28-31(27,29-4-2)8-18-10(22)7-17-14(25)13-11(23)12(24)15(30-13)20-6-5-9(21)19-16(20)26/h5-6,11-13,15,23-24H,3-4,7-8H2,1-2H3,(H,17,25)(H,18,22)(H,19,21,26)/t11-,12+,13-,15+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human NTPDase 1 expressed in COS7 cells |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CD39 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50546280
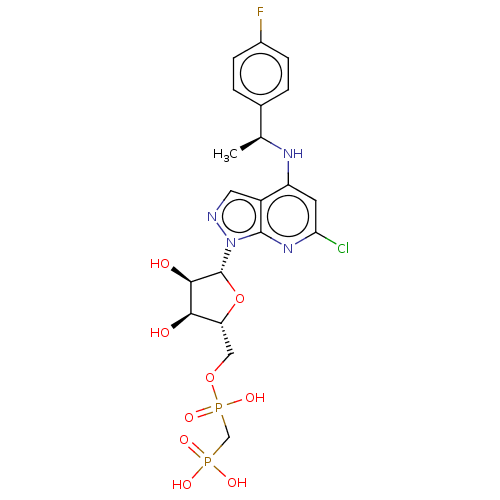
(CHEMBL4761506)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccc(F)cc1 |r| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CD39 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50546288

(CHEMBL4743437)Show SMILES C[C@H](Nc1nc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1 |r| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CD39 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50615669
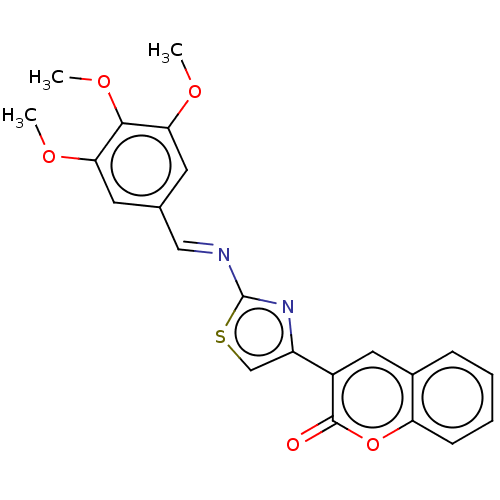
(CHEMBL5277365) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Mus musculus) | BDBM4078
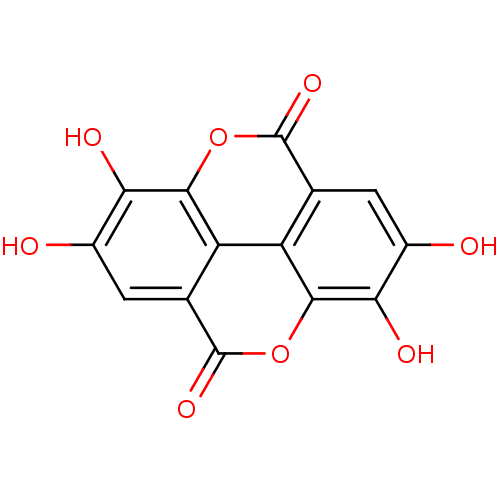
(6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...)Show InChI InChI=1S/C14H6O8/c15-5-1-3-7-8-4(14(20)22-11(7)9(5)17)2-6(16)10(18)12(8)21-13(3)19/h1-2,15-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse CD39 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127758
BindingDB Entry DOI: 10.7270/Q2GM8BZW |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50615668
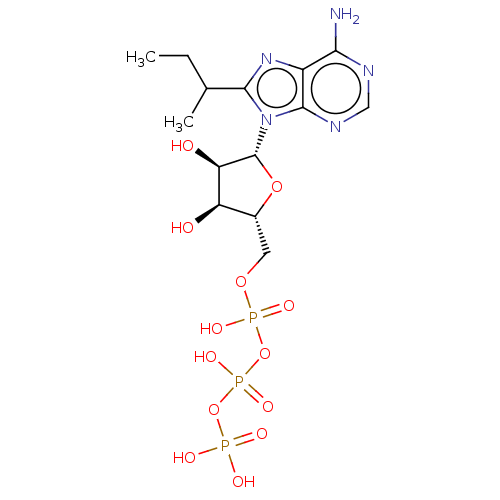
(CHEMBL5283491) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50468622
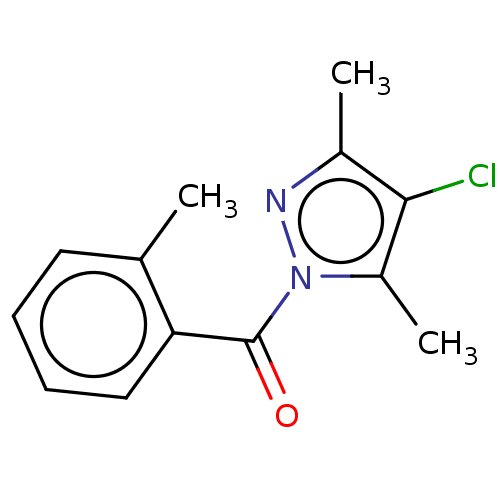
(CHEMBL4285649)Show InChI InChI=1S/C13H13ClN2O/c1-8-6-4-5-7-11(8)13(17)16-10(3)12(14)9(2)15-16/h4-7H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase1 expressed in CHO cells using ATP as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi... |
Eur J Med Chem 156: 461-478 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.002
BindingDB Entry DOI: 10.7270/Q21C20K3 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
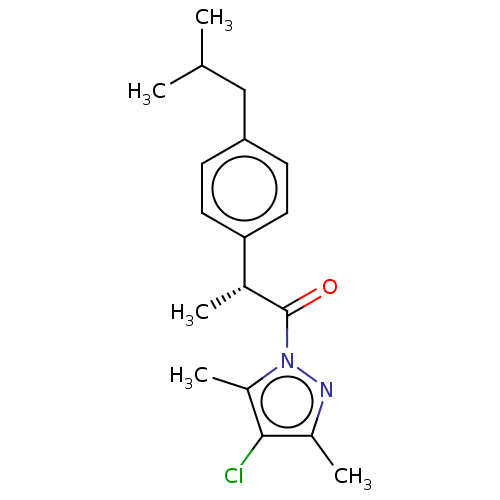
(Rattus norvegicus) | BDBM50468632
(CHEMBL4293491)Show SMILES CC(C)Cc1ccc(cc1)[C@@H](C)C(=O)n1nc(C)c(Cl)c1C |r| Show InChI InChI=1S/C18H23ClN2O/c1-11(2)10-15-6-8-16(9-7-15)12(3)18(22)21-14(5)17(19)13(4)20-21/h6-9,11-12H,10H2,1-5H3/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase1 expressed in CHO cells using ATP as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi... |
Eur J Med Chem 156: 461-478 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.002
BindingDB Entry DOI: 10.7270/Q21C20K3 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Mus musculus) | BDBM50241052

(1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33-,34+,35-,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse CD39 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127758
BindingDB Entry DOI: 10.7270/Q2GM8BZW |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
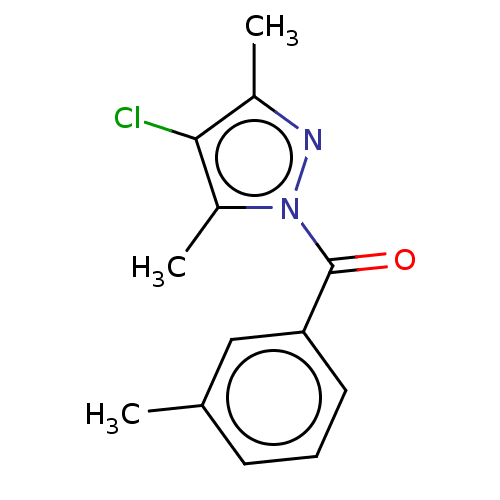
(Rattus norvegicus) | BDBM50468637
(CHEMBL4295005)Show InChI InChI=1S/C13H13ClN2O/c1-8-5-4-6-11(7-8)13(17)16-10(3)12(14)9(2)15-16/h4-7H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase1 expressed in CHO cells using ATP as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi... |
Eur J Med Chem 156: 461-478 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.002
BindingDB Entry DOI: 10.7270/Q21C20K3 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50552128

(CHEMBL4787368)Show SMILES OS(=O)(=O)C1=CC\C(=N/C(=O)C2=C\C(C=CC2)=N\C(=O)C2=CC=C\C(C2)=N/C(=O)/N=C2/CC(=CC=C2)C(=O)\N=C2\CC(=CC=C2)C(=O)\N=C2/CC=C(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c2c1cc(cc2S(O)(=O)=O)S(O)(=O)=O |c:14,23,34,36,44,46,54,t:4,11,21| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 expressed in green monkey Cos-7 cells using ATP as substrate preincubated for 3 mins followed by substrate addition by malac... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50010311

(CHEMBL2364580)Show SMILES CCCCSc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H24N5O13P3S/c1-2-3-4-36-14-18-8-11(15)16-6-17-12(8)19(14)13-10(21)9(20)7(30-13)5-29-34(25,26)32-35(27,28)31-33(22,23)24/h6-7,9-10,13,20-21H,2-5H2,1H3,(H,25,26)(H,27,28)(H2,15,16,17)(H2,22,23,24)/t7-,9-,10-,13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 using ATP as substrate preincubated for 3 mins followed by substrate addition and measured after 10 to 15 mins by Malachite ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50468623
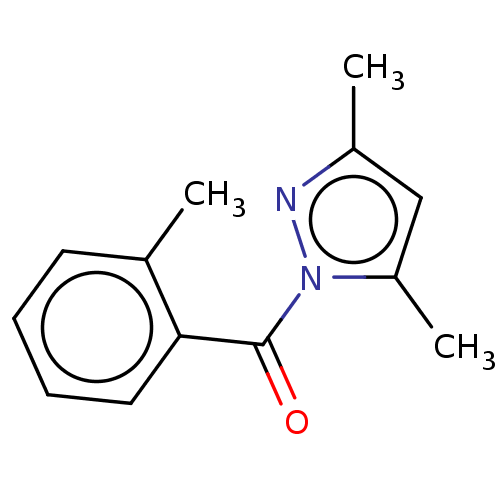
(CHEMBL4290501)Show InChI InChI=1S/C13H14N2O/c1-9-6-4-5-7-12(9)13(16)15-11(3)8-10(2)14-15/h4-8H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase1 expressed in CHO cells using ATP as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi... |
Eur J Med Chem 156: 461-478 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.002
BindingDB Entry DOI: 10.7270/Q21C20K3 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
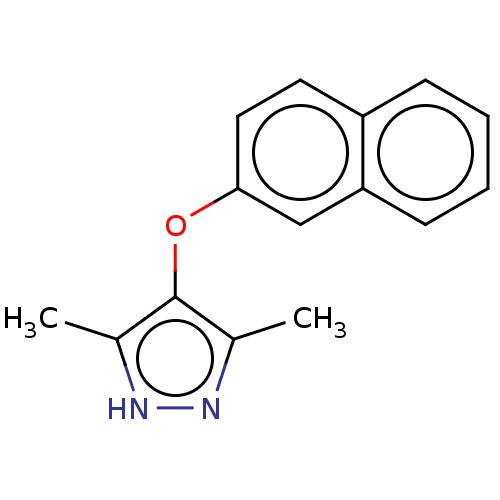
(Rattus norvegicus) | BDBM50468631
(CHEMBL4291645)Show InChI InChI=1S/C15H14N2O/c1-10-15(11(2)17-16-10)18-14-8-7-12-5-3-4-6-13(12)9-14/h3-9H,1-2H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase1 expressed in CHO cells using ATP as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi... |
Eur J Med Chem 156: 461-478 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.002
BindingDB Entry DOI: 10.7270/Q21C20K3 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50561221

(CHEMBL4745473 | US11377469, Example 95)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COCP(O)(O)=O)n1ncc2c(NC3CCCC3)nc(Cl)nc12 |r| | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 expressed in CHO cells incubated for 4 hrs before addition of AMP and further incubated for 60 mins by colorimetric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01835
BindingDB Entry DOI: 10.7270/Q2NG4VF1 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
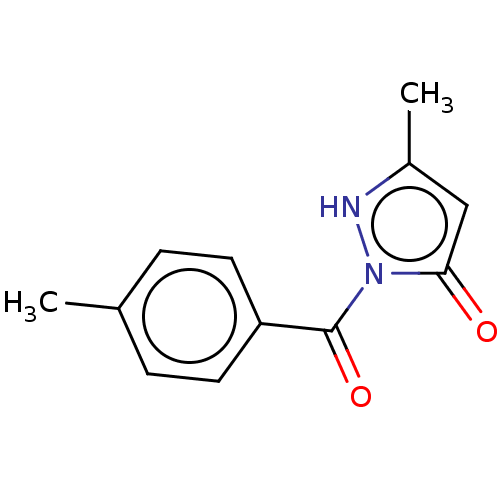
(Rattus norvegicus) | BDBM50468629
(CHEMBL4279910)Show InChI InChI=1S/C12H12N2O2/c1-8-3-5-10(6-4-8)12(16)14-11(15)7-9(2)13-14/h3-7,13H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-I-Azam University
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase1 expressed in CHO cells using ATP as substrate preincubated for 10 mins followed by substrate addition measured after 10 mi... |
Eur J Med Chem 156: 461-478 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.002
BindingDB Entry DOI: 10.7270/Q21C20K3 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CD39 expressed in green monkey Cos-7 cells using ATP as substrate preincubated for 3 mins followed by substrate addition by malac... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Rostock
Curated by ChEMBL
| Assay Description
Inhibition of human NTPDase1 expressed in African green monkey COS7 cell membrane fraction using ATP as substrate preincubated for 10 mins followed b... |
Eur J Med Chem 138: 816-829 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.017
BindingDB Entry DOI: 10.7270/Q20R9RXT |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
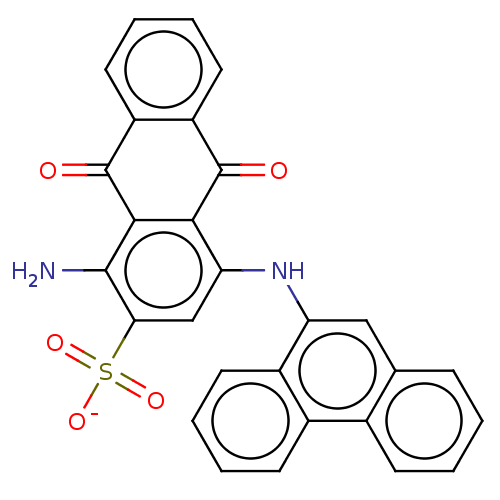
(Homo sapiens (Human)) | BDBM50187685
(CHEMBL597418)Show SMILES [Na+].Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2cc3ccccc3c3ccccc23)cc1S([O-])(=O)=O Show InChI InChI=1S/C28H18N2O5S.Na/c29-26-23(36(33,34)35)14-22(24-25(26)28(32)20-12-6-5-11-19(20)27(24)31)30-21-13-15-7-1-2-8-16(15)17-9-3-4-10-18(17)21;/h1-14,30H,29H2,(H,33,34,35);/q;+1/p-1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human NTPDase1 expressed in CHO cells using ADP as substrate incubated for 10 mins by capillary electrophoresis method |
Bioorg Med Chem 24: 4363-4371 (2016)
Article DOI: 10.1016/j.bmc.2016.07.027
BindingDB Entry DOI: 10.7270/Q2GQ70Q8 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Homo sapiens (Human)) | BDBM50527649

(CHEMBL4467828)Show SMILES Clc1cc(cc2n(nnc12)-c1ccc2cn[nH]c2c1)-c1ccnn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C24H15ClN8/c25-20-9-18(22-7-8-28-32(22)14-16-3-1-15(12-26)2-4-16)10-23-24(20)30-31-33(23)19-6-5-17-13-27-29-21(17)11-19/h1-11,13H,14H2,(H,27,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CD39 expressed in CHO cells using ATP as substrate preincubated for 1 hr followed by substrate addition and measured after 50 min... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data