

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 288.3 BDBM50262140 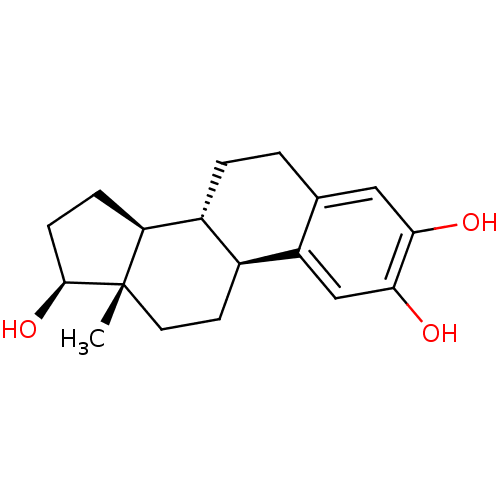 Purchase Purchase | Wt: 280.7 BDBM492902 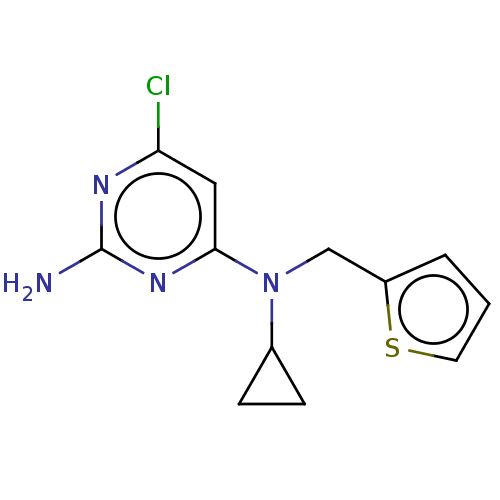 Purchase Purchase | Wt: 240.3 BDBM492904  Purchase Purchase | Wt: 317.7 BDBM50577233 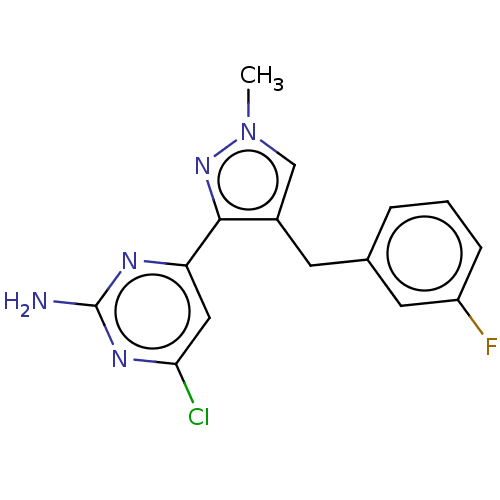 | Wt: 299.7 BDBM50577237 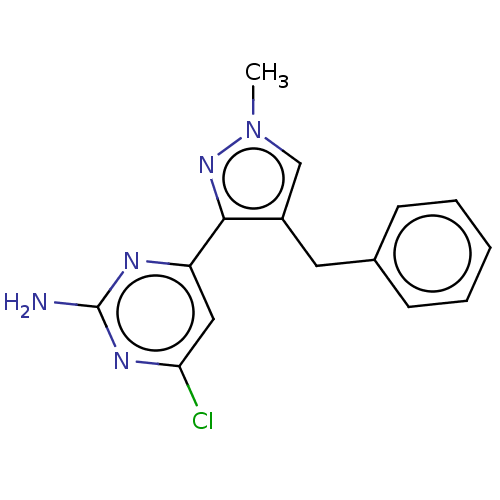 |
| Wt: 317.7 BDBM50577236 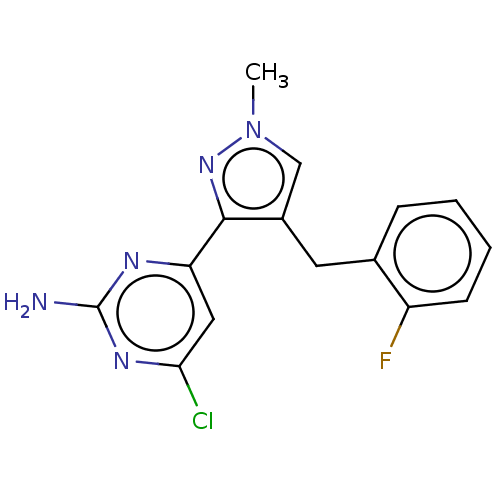 | Wt: 306.7 BDBM50577238 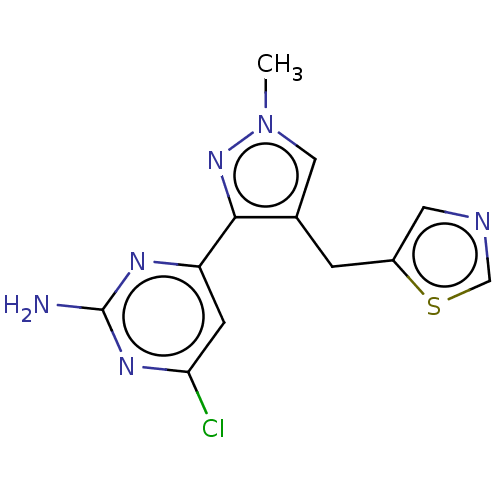 | Wt: 285.3 BDBM50577240 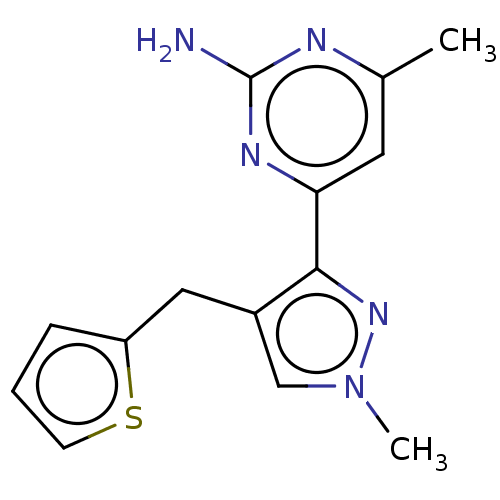 | Wt: 319.8 BDBM50577241 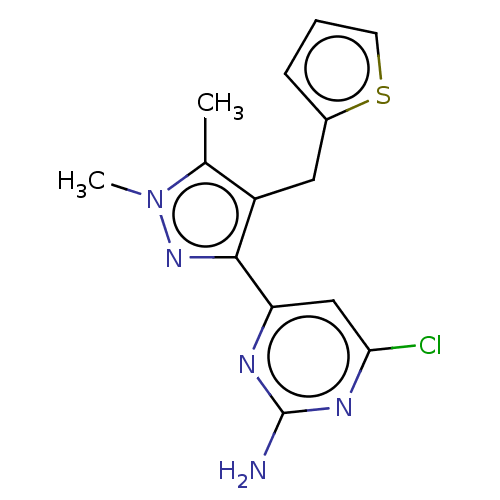 | Wt: 313.7 BDBM50577242 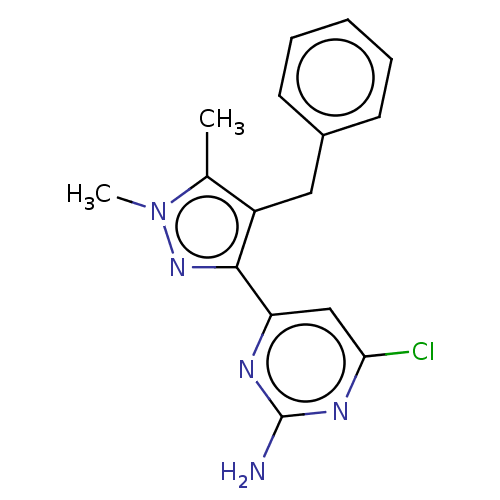 Purchase Purchase |
| Wt: 303.7 BDBM50577245 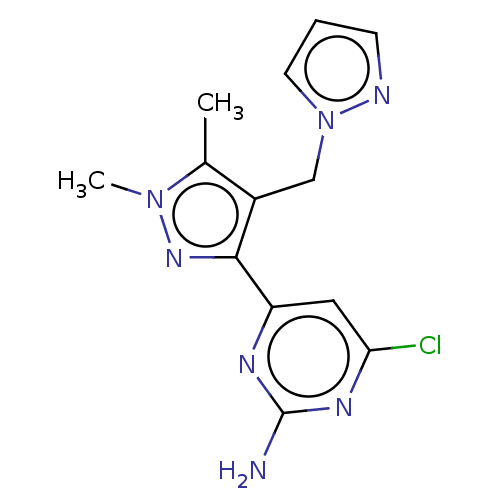 | Wt: 317.7 BDBM50577246 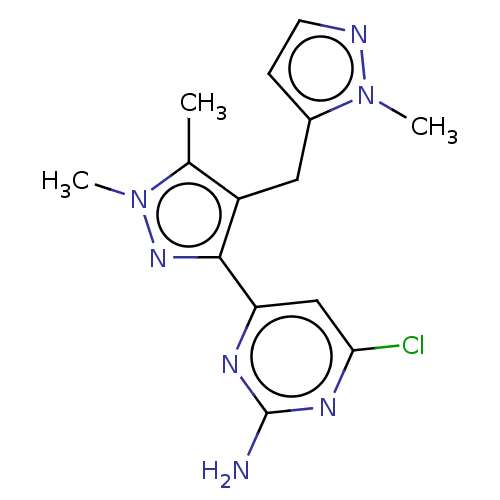 | Wt: 315.7 BDBM50577247 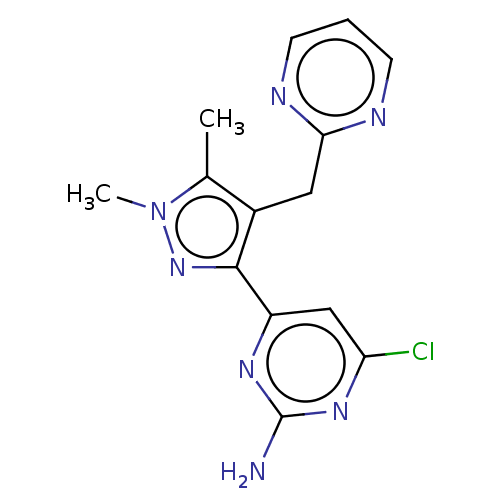 | Wt: 305.7 BDBM50577232 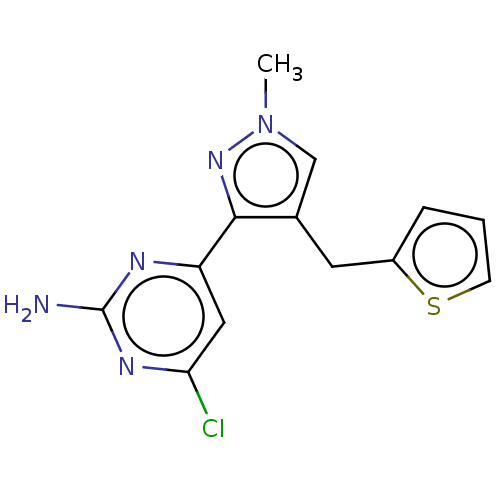 | Wt: 317.7 BDBM50577234 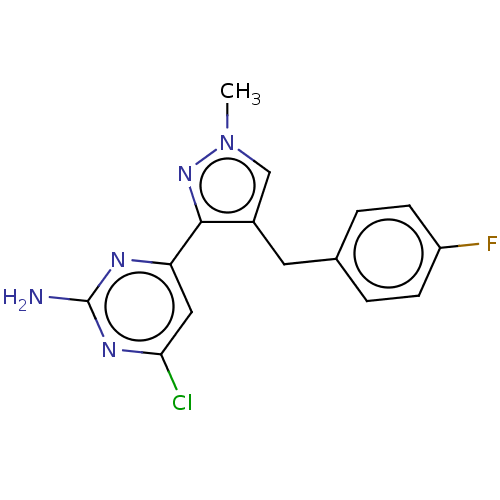 |
| Displayed 1 to 15 (of 58 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disabled homolog 2 (Human) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TRUSTEES OF DARTMOUTH COLLEGE US Patent | Assay Description Fluorescence polarization (FP) data were measured on a microplate reader (Tecan Infinite M1000, Mannedorf, Switzerland) at 27° C. For Kd measurements... | US Patent US10864248 (2020) BindingDB Entry DOI: 10.7270/Q2R214GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577241 (CHEMBL4848035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577242 (CHEMBL4854762) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577241 (CHEMBL4848035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577236 (CHEMBL4857305) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577232 (CHEMBL4849033) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577242 (CHEMBL4854762) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01133 BindingDB Entry DOI: 10.7270/Q2057M2B | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577242 (CHEMBL4854762) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01133 BindingDB Entry DOI: 10.7270/Q2057M2B | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577242 (CHEMBL4854762) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577232 (CHEMBL4849033) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577233 (CHEMBL4874288) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577245 (CHEMBL4866319) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577234 (CHEMBL4864843) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577233 (CHEMBL4874288) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577238 (CHEMBL4866035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 576 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577237 (CHEMBL4874934) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 989 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577240 (CHEMBL4847756) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577237 (CHEMBL4874934) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01133 BindingDB Entry DOI: 10.7270/Q2057M2B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492902 (US10981899, Example RU-0204277-LRE1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ruhr-University Bochum Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble adenylyl cyclase | J Med Chem 51: 4456-64 (2008) Article DOI: 10.1021/jm800481q BindingDB Entry DOI: 10.7270/Q22N5355 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492902 (US10981899, Example RU-0204277-LRE1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Tri-Institutional Therapeutics Discovery Institute US Patent | Assay Description The RapidFire 365 High-throughput MS System (Agilent Technologies; RF-MSS) can process samples every 15 seconds allowing analysis of a 384 well plate... | US Patent US10981899 (2021) BindingDB Entry DOI: 10.7270/Q2WD43Q0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492902 (US10981899, Example RU-0204277-LRE1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Tri-Institutional Therapeutics Discovery Institute US Patent | Assay Description INS-1E insulinoma cells were incubated in 2.5 mM glucose Krebs-Ringer buffer (pH 7.5) supplemented with 2 mM sodium bicarbonate, 10 mM HEPES, and 0.1... | US Patent US10981899 (2021) BindingDB Entry DOI: 10.7270/Q2WD43Q0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881) | J Med Chem 48: 5666-74 (2005) Article DOI: 10.1021/jm050403f BindingDB Entry DOI: 10.7270/Q2TM7CBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577246 (CHEMBL4851867) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577247 (CHEMBL4874074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492904 (US10981899, Example RU-0207328) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Tri-Institutional Therapeutics Discovery Institute US Patent | Assay Description The RapidFire 365 High-throughput MS System (Agilent Technologies; RF-MSS) can process samples every 15 seconds allowing analysis of a 384 well plate... | US Patent US10981899 (2021) BindingDB Entry DOI: 10.7270/Q2WD43Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492904 (US10981899, Example RU-0207328) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Tri-Institutional Therapeutics Discovery Institute US Patent | Assay Description INS-1E insulinoma cells were incubated in 2.5 mM glucose Krebs-Ringer buffer (pH 7.5) supplemented with 2 mM sodium bicarbonate, 10 mM HEPES, and 0.1... | US Patent US10981899 (2021) BindingDB Entry DOI: 10.7270/Q2WD43Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase (Plasmodium falciparum 3D7) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2ZS2TZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase (Plasmodium falciparum 3D7) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q23T9FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase (Plasmodium falciparum 3D7) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q27H1H3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 7.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2V12389 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock factor protein 1 (Mus musculus) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Keywords: Heat Shock Factor-1 (HSF-1), Stress Response, MG132, NIH3T3, Luminescence Assay Overview: Confirmation testing of small molecules identifie... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2P55KXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Affinity estrogen receptor of MCF-7 human mammary cancer cells | J Med Chem 39: 1917-23 (1996) Article DOI: 10.1021/jm9508245 BindingDB Entry DOI: 10.7270/Q21G0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM50577242 (CHEMBL4854762) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01133 BindingDB Entry DOI: 10.7270/Q2057M2B | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50262140 ((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 504 | n/a | n/a | n/a | n/a |
Ohio State University Curated by ChEMBL | Assay Description Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells | J Med Chem 39: 1917-23 (1996) Article DOI: 10.1021/jm9508245 BindingDB Entry DOI: 10.7270/Q21G0Q0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||