 Found 1269 hits with Last Name = 'huang' and Initial = 'a'
Found 1269 hits with Last Name = 'huang' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510
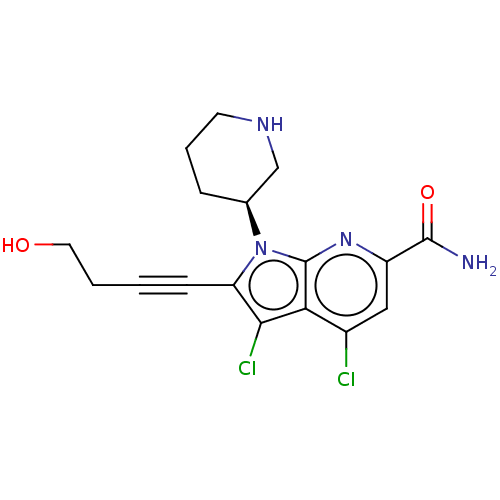
(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517
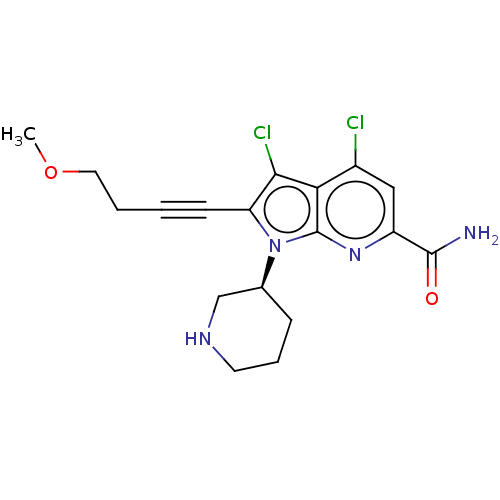
(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518507

(CHEMBL4583118)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CNCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H21Cl2N5O2/c20-13-8-14(18(22)27)25-19-16(13)17(21)15(4-3-12-10-24-6-7-28-12)26(19)11-2-1-5-23-9-11/h8,11-12,23-24H,1-2,5-7,9-10H2,(H2,22,27)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50302828
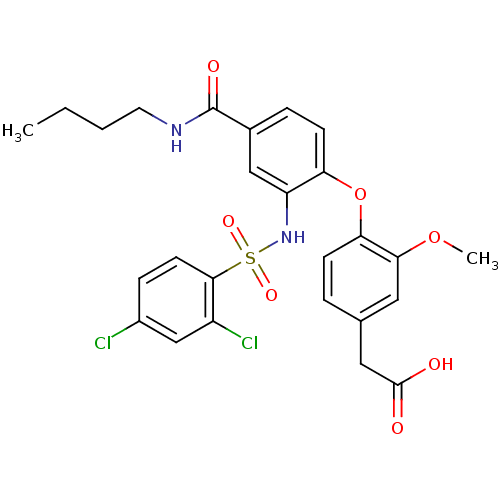
(2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...)Show SMILES CCCCNC(=O)c1ccc(Oc2ccc(CC(O)=O)cc2OC)c(NS(=O)(=O)c2ccc(Cl)cc2Cl)c1 Show InChI InChI=1S/C26H26Cl2N2O7S/c1-3-4-11-29-26(33)17-6-9-21(37-22-8-5-16(13-25(31)32)12-23(22)36-2)20(14-17)30-38(34,35)24-10-7-18(27)15-19(24)28/h5-10,12,14-15,30H,3-4,11,13H2,1-2H3,(H,29,33)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PGD2-induced CRTH2 receptor internalization of CD16 negative granulocytes in human whole blood by flow cytometry |
Bioorg Med Chem Lett 19: 6419-23 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.052
BindingDB Entry DOI: 10.7270/Q25Q4X20 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518518
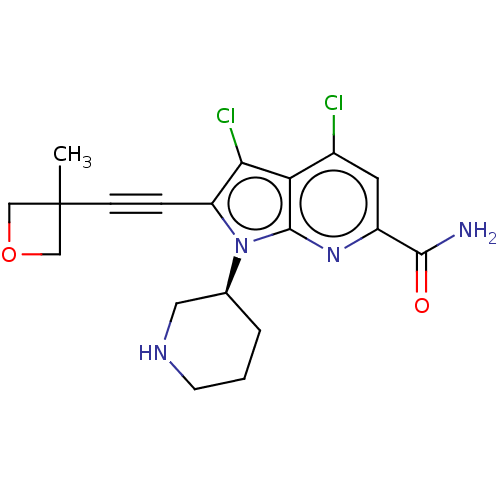
(CHEMBL4470576)Show SMILES CC1(COC1)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C19H20Cl2N4O2/c1-19(9-27-10-19)5-4-14-16(21)15-12(20)7-13(17(22)26)24-18(15)25(14)11-3-2-6-23-8-11/h7,11,23H,2-3,6,8-10H2,1H3,(H2,22,26)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518514
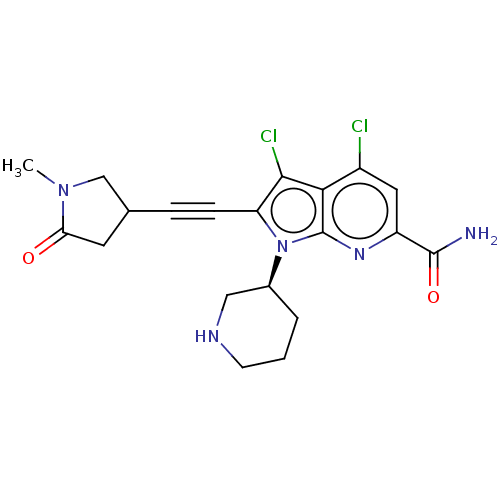
(CHEMBL4563802)Show SMILES CN1CC(CC1=O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H21Cl2N5O2/c1-26-10-11(7-16(26)28)4-5-15-18(22)17-13(21)8-14(19(23)29)25-20(17)27(15)12-3-2-6-24-9-12/h8,11-12,24H,2-3,6-7,9-10H2,1H3,(H2,23,29)/t11?,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518505
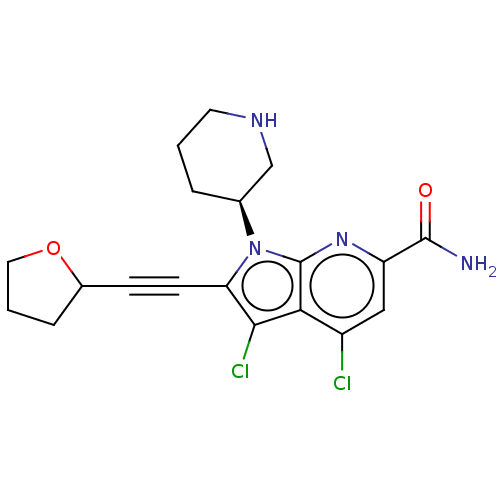
(CHEMBL4518492)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O2/c20-13-9-14(18(22)26)24-19-16(13)17(21)15(6-5-12-4-2-8-27-12)25(19)11-3-1-7-23-10-11/h9,11-12,23H,1-4,7-8,10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518522

(CHEMBL4466080)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C16H16Cl2N4O2/c17-10-7-11(15(19)24)21-16-13(10)14(18)12(4-2-6-23)22(16)9-3-1-5-20-8-9/h7,9,20,23H,1,3,5-6,8H2,(H2,19,24)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233

(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES C[C@H](OC(C)(C)C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C=O |r| Show InChI InChI=1S/C32H48N4O7/c1-21(43-32(2,3)4)27(36-31(41)42-20-23-13-9-6-10-14-23)30(40)35-26(17-22-11-7-5-8-12-22)29(39)34-25(19-37)18-24-15-16-33-28(24)38/h6,9-10,13-14,19,21-22,24-27H,5,7-8,11-12,15-18,20H2,1-4H3,(H,33,38)(H,34,39)(H,35,40)(H,36,41)/t21-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232
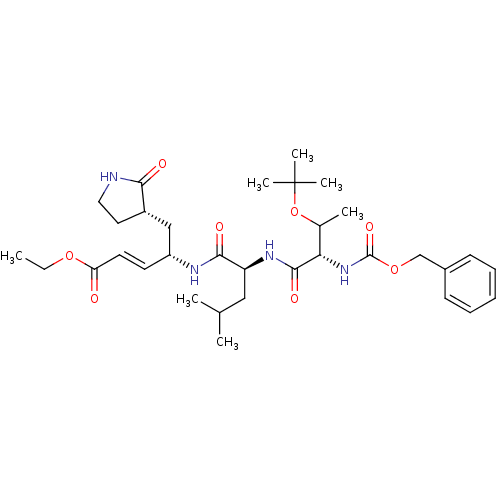
(N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)OC(C)(C)C |r| Show InChI InChI=1S/C33H50N4O8/c1-8-43-27(38)15-14-25(19-24-16-17-34-29(24)39)35-30(40)26(18-21(2)3)36-31(41)28(22(4)45-33(5,6)7)37-32(42)44-20-23-12-10-9-11-13-23/h9-15,21-22,24-26,28H,8,16-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b15-14+/t22?,24-,25+,26-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50302828

(2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...)Show SMILES CCCCNC(=O)c1ccc(Oc2ccc(CC(O)=O)cc2OC)c(NS(=O)(=O)c2ccc(Cl)cc2Cl)c1 Show InChI InChI=1S/C26H26Cl2N2O7S/c1-3-4-11-29-26(33)17-6-9-21(37-22-8-5-16(13-25(31)32)12-23(22)36-2)20(14-17)30-38(34,35)24-10-7-18(27)15-19(24)28/h5-10,12,14-15,30H,3-4,11,13H2,1-2H3,(H,29,33)(H,31,32) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP receptor in human platelets assessed as inhibition of PGD2-induced cAMP production by competitive ELISA |
Bioorg Med Chem Lett 19: 6419-23 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.052
BindingDB Entry DOI: 10.7270/Q25Q4X20 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231

(N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C30H44N4O7/c1-6-40-25(35)13-12-23(17-22-14-15-31-27(22)36)32-28(37)24(16-19(2)3)33-29(38)26(20(4)5)34-30(39)41-18-21-10-8-7-9-11-21/h7-13,19-20,22-24,26H,6,14-18H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H,34,39)/b13-12+/t22-,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230
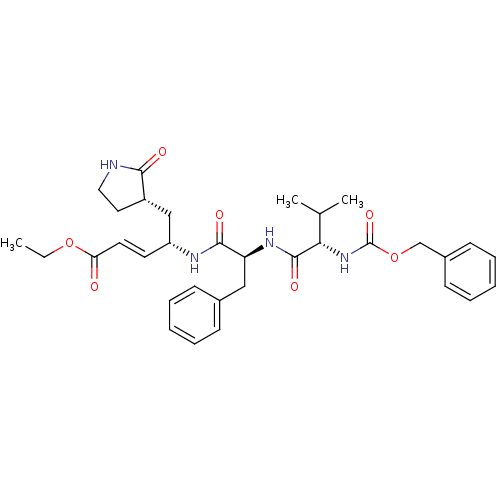
(AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C33H42N4O7/c1-4-43-28(38)16-15-26(20-25-17-18-34-30(25)39)35-31(40)27(19-23-11-7-5-8-12-23)36-32(41)29(22(2)3)37-33(42)44-21-24-13-9-6-10-14-24/h5-16,22,25-27,29H,4,17-21H2,1-3H3,(H,34,39)(H,35,40)(H,36,41)(H,37,42)/b16-15+/t25-,26+,27-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229
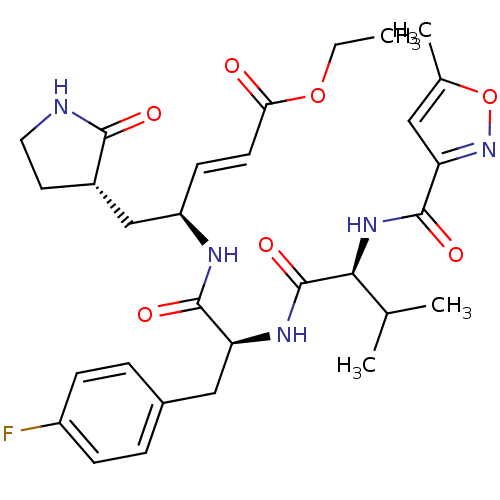
(AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...)Show SMILES CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)[C@@H](NC(=O)c1cc(C)on1)C(C)C |r| Show InChI InChI=1S/C30H38FN5O7/c1-5-42-25(37)11-10-22(16-20-12-13-32-27(20)38)33-28(39)23(15-19-6-8-21(31)9-7-19)34-30(41)26(17(2)3)35-29(40)24-14-18(4)43-36-24/h6-11,14,17,20,22-23,26H,5,12-13,15-16H2,1-4H3,(H,32,38)(H,33,39)(H,34,41)(H,35,40)/b11-10+/t20-,22+,23-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co.
| Assay Description
The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... |
J Med Chem 49: 4971-80 (2006)
Article DOI: 10.1021/jm0603926
BindingDB Entry DOI: 10.7270/Q24B2ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50302828

(2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...)Show SMILES CCCCNC(=O)c1ccc(Oc2ccc(CC(O)=O)cc2OC)c(NS(=O)(=O)c2ccc(Cl)cc2Cl)c1 Show InChI InChI=1S/C26H26Cl2N2O7S/c1-3-4-11-29-26(33)17-6-9-21(37-22-8-5-16(13-25(31)32)12-23(22)36-2)20(14-17)30-38(34,35)24-10-7-18(27)15-19(24)28/h5-10,12,14-15,30H,3-4,11,13H2,1-2H3,(H,29,33)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EP2 expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP production |
Bioorg Med Chem Lett 19: 6419-23 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.052
BindingDB Entry DOI: 10.7270/Q25Q4X20 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50302828

(2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...)Show SMILES CCCCNC(=O)c1ccc(Oc2ccc(CC(O)=O)cc2OC)c(NS(=O)(=O)c2ccc(Cl)cc2Cl)c1 Show InChI InChI=1S/C26H26Cl2N2O7S/c1-3-4-11-29-26(33)17-6-9-21(37-22-8-5-16(13-25(31)32)12-23(22)36-2)20(14-17)30-38(34,35)24-10-7-18(27)15-19(24)28/h5-10,12,14-15,30H,3-4,11,13H2,1-2H3,(H,29,33)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EP4 expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP production |
Bioorg Med Chem Lett 19: 6419-23 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.052
BindingDB Entry DOI: 10.7270/Q25Q4X20 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341209
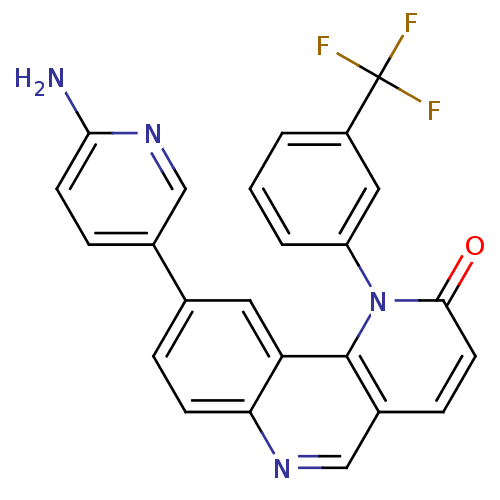
(9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phen...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4cccc(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510

(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554405
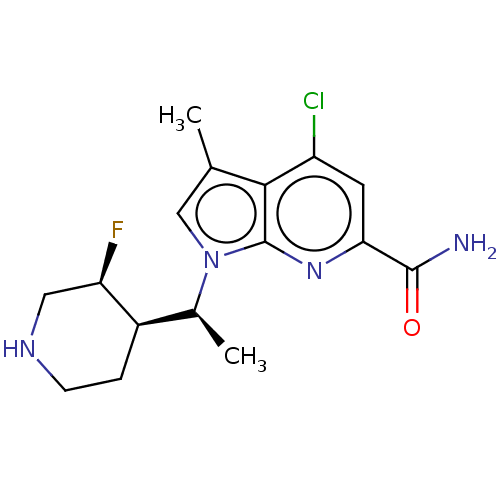
(CHEMBL4759685)Show SMILES [H][C@@]1(CCNC[C@H]1F)[C@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by ADP-gloassay |
Eur J Med Chem 164: 304-316 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.055
BindingDB Entry DOI: 10.7270/Q23J3HF7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM317549
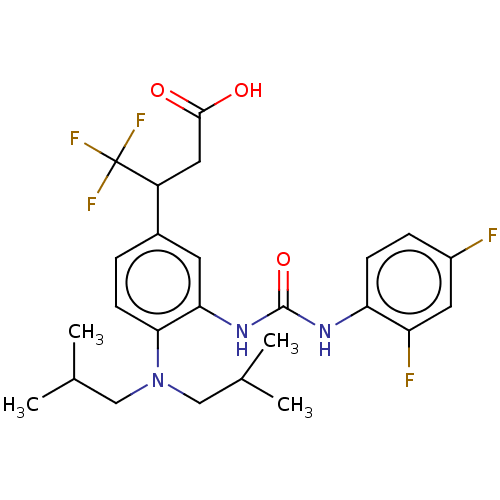
(3-(3-(3-(2,4- difluorophenyl)ureido)-4- (diisobuty...)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(F)cc1F)C(CC(O)=O)C(F)(F)F Show InChI InChI=1S/C25H30F5N3O3/c1-14(2)12-33(13-15(3)4)22-8-5-16(18(11-23(34)35)25(28,29)30)9-21(22)32-24(36)31-20-7-6-17(26)10-19(20)27/h5-10,14-15,18H,11-13H2,1-4H3,(H,34,35)(H2,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... |
US Patent US9624188 (2017)
BindingDB Entry DOI: 10.7270/Q2GF0WKT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518521

(CHEMBL4564586)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C18H18Cl2N4O/c19-12-8-13(17(21)25)23-18-15(12)16(20)14(6-5-10-3-4-10)24(18)11-2-1-7-22-9-11/h8,10-11,22H,1-4,7,9H2,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50006915

(CHEMBL3236929)Show SMILES Cc1c(Cc2ccc(=O)n(Cc3cccc(F)c3F)c2)c2cc(F)ccc2n1CC(O)=O Show InChI InChI=1S/C24H19F3N2O3/c1-14-18(19-10-17(25)6-7-21(19)29(14)13-23(31)32)9-15-5-8-22(30)28(11-15)12-16-3-2-4-20(26)24(16)27/h2-8,10-11H,9,12-13H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CRTh2 expressed in CHO-K1 cells by cAMP TR FRET assay |
J Med Chem 57: 1299-322 (2014)
Article DOI: 10.1021/jm401509e
BindingDB Entry DOI: 10.7270/Q21V5GGH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50401116
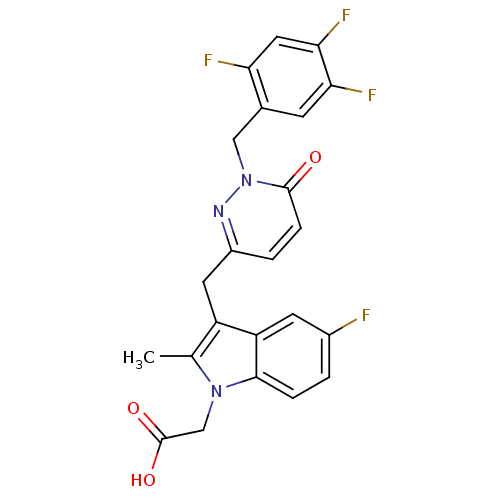
(CHEMBL2204474)Show SMILES Cc1c(Cc2ccc(=O)n(Cc3cc(F)c(F)cc3F)n2)c2cc(F)ccc2n1CC(O)=O Show InChI InChI=1S/C23H17F4N3O3/c1-12-16(17-7-14(24)2-4-21(17)29(12)11-23(32)33)8-15-3-5-22(31)30(28-15)10-13-6-19(26)20(27)9-18(13)25/h2-7,9H,8,10-11H2,1H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from recombinant human CRTH2 receptor expressed in CHO-K1 cells after 30 min by FRET method |
J Med Chem 55: 5088-109 (2012)
Article DOI: 10.1021/jm300007n
BindingDB Entry DOI: 10.7270/Q2S75HH0 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50401097
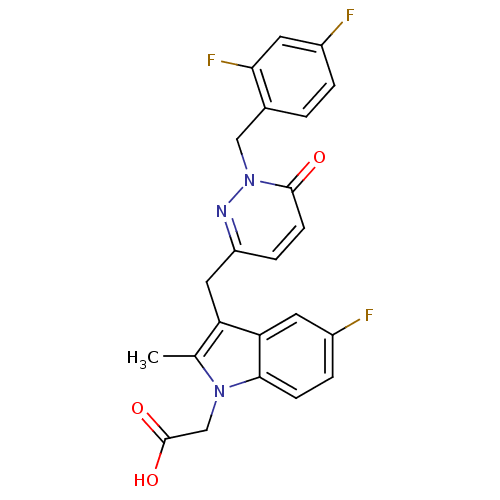
(CHEMBL2204469)Show SMILES Cc1c(Cc2ccc(=O)n(Cc3ccc(F)cc3F)n2)c2cc(F)ccc2n1CC(O)=O Show InChI InChI=1S/C23H18F3N3O3/c1-13-18(19-8-15(24)4-6-21(19)28(13)12-23(31)32)10-17-5-7-22(30)29(27-17)11-14-2-3-16(25)9-20(14)26/h2-9H,10-12H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CRTH2 receptor |
J Med Chem 55: 5088-109 (2012)
Article DOI: 10.1021/jm300007n
BindingDB Entry DOI: 10.7270/Q2S75HH0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517

(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM144614
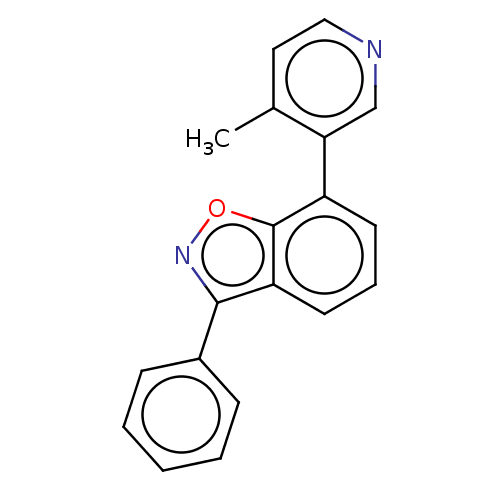
(US8969586, 1 | US9598436, 1)Show InChI InChI=1S/C19H14N2O/c1-13-10-11-20-12-17(13)15-8-5-9-16-18(21-22-19(15)16)14-6-3-2-4-7-14/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM317558
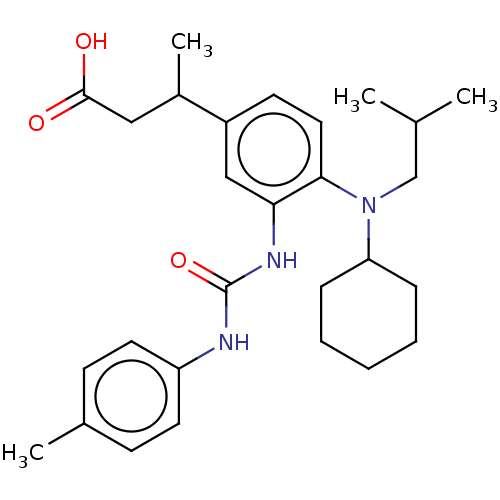
(US9624188, 49 | racemic)Show SMILES CC(C)CN(C1CCCCC1)c1ccc(cc1NC(=O)Nc1ccc(C)cc1)C(C)CC(O)=O Show InChI InChI=1S/C28H39N3O3/c1-19(2)18-31(24-8-6-5-7-9-24)26-15-12-22(21(4)16-27(32)33)17-25(26)30-28(34)29-23-13-10-20(3)11-14-23/h10-15,17,19,21,24H,5-9,16,18H2,1-4H3,(H,32,33)(H2,29,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... |
US Patent US9624188 (2017)
BindingDB Entry DOI: 10.7270/Q2GF0WKT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554401
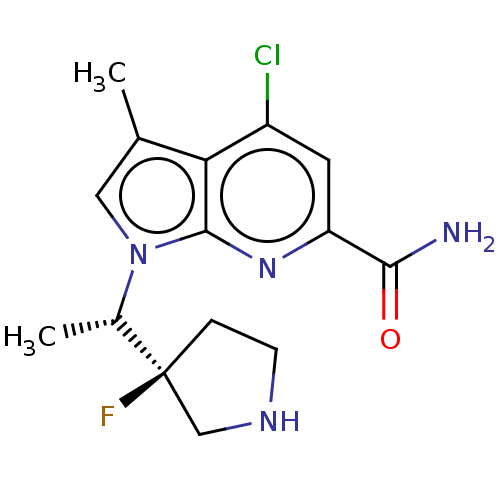
(CHEMBL4778578)Show SMILES C[C@H](n1cc(C)c2c(Cl)cc(nc12)C(N)=O)[C@]1(F)CCNC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal His tagged BTK expressed in baculovirus infected Sf9 cells using poly (4:1 Glu, Tyr) as substr... |
Bioorg Med Chem 26: 4537-4543 (2018)
Article DOI: 10.1016/j.bmc.2018.07.047
BindingDB Entry DOI: 10.7270/Q20C4ZDW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554403
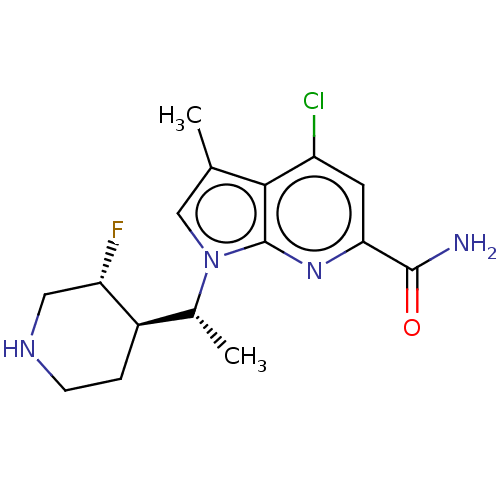
(CHEMBL4750046)Show SMILES [H][C@@]1(CCNC[C@@H]1F)[C@@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554404
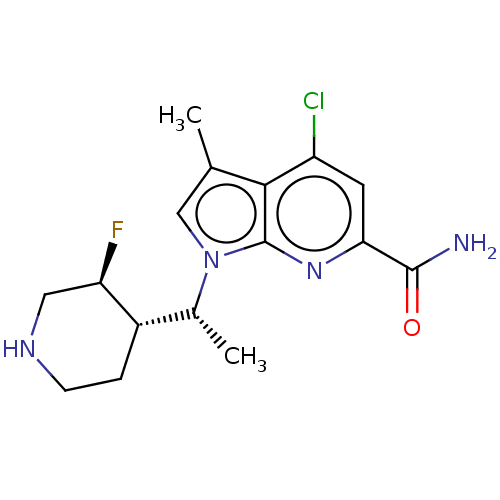
(CHEMBL4745141)Show SMILES [H][C@]1(CCNC[C@H]1F)[C@@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM23508
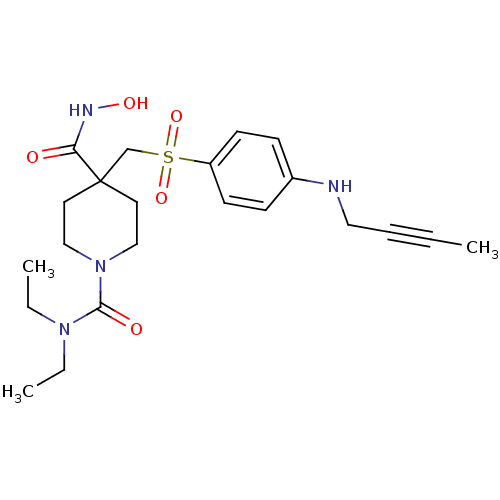
(4-({[4-(but-2-yn-1-ylamino)benzene]sulfonyl}methyl...)Show SMILES CCN(CC)C(=O)N1CCC(CS(=O)(=O)c2ccc(NCC#CC)cc2)(CC1)C(=O)NO Show InChI InChI=1S/C22H32N4O5S/c1-4-7-14-23-18-8-10-19(11-9-18)32(30,31)17-22(20(27)24-29)12-15-26(16-13-22)21(28)25(5-2)6-3/h8-11,23,29H,5-6,12-17H2,1-3H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... |
Bioorg Med Chem 15: 6170-81 (2007)
Article DOI: 10.1016/j.bmc.2007.06.031
BindingDB Entry DOI: 10.7270/Q2FJ2F3N |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50006823
(CHEMBL3236950)Show SMILES Cc1c(-c2cn(CCCC(F)(F)F)c(=O)c3ccccc23)c2cc(F)ccc2n1CC(O)=O |(20.58,-56.23,;19.11,-56.7,;17.87,-55.8,;17.87,-54.26,;19.21,-53.49,;19.21,-51.95,;20.54,-51.18,;21.88,-51.96,;23.21,-51.19,;24.55,-51.96,;25.88,-51.19,;24.55,-53.5,;26.03,-52.36,;17.87,-51.18,;17.87,-49.64,;16.54,-51.96,;15.21,-51.19,;13.88,-51.96,;13.89,-53.5,;15.22,-54.26,;16.54,-53.49,;16.62,-56.71,;15.12,-56.39,;14.1,-57.53,;12.59,-57.21,;14.57,-58.99,;16.07,-59.3,;17.09,-58.17,;18.63,-58.17,;19.54,-59.42,;21.07,-59.26,;21.7,-57.85,;21.98,-60.5,)| Show InChI InChI=1S/C24H20F4N2O3/c1-14-22(18-11-15(25)7-8-20(18)30(14)13-21(31)32)19-12-29(10-4-9-24(26,27)28)23(33)17-6-3-2-5-16(17)19/h2-3,5-8,11-12H,4,9-10,13H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CRTh2 expressed in CHO-K1 cells by cAMP TR FRET assay |
J Med Chem 57: 1299-322 (2014)
Article DOI: 10.1021/jm401509e
BindingDB Entry DOI: 10.7270/Q21V5GGH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM144614

(US8969586, 1 | US9598436, 1)Show InChI InChI=1S/C19H14N2O/c1-13-10-11-20-12-17(13)15-8-5-9-16-18(21-22-19(15)16)14-6-3-2-4-7-14/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The assays were performed in U-bottom 384-well optiplates. The final assay volume was 15 μl prepared from 7.5 μl additions of microsomes (p... |
US Patent US8969586 (2015)
BindingDB Entry DOI: 10.7270/Q2MW2FVH |
More data for this
Ligand-Target Pair | |
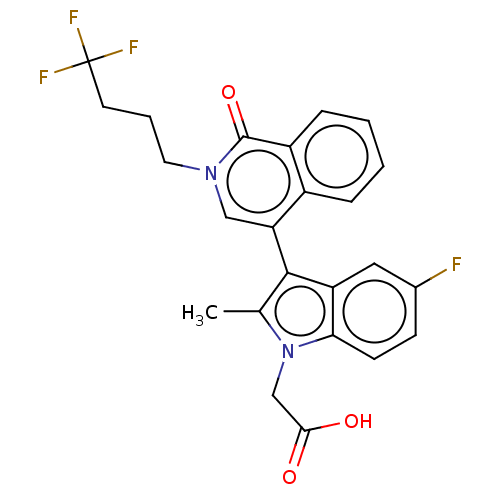
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50518511
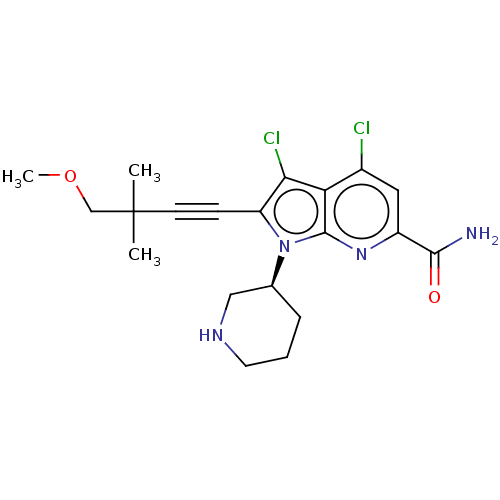
(CHEMBL4457529)Show SMILES COCC(C)(C)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H24Cl2N4O2/c1-20(2,11-28-3)7-6-15-17(22)16-13(21)9-14(18(23)27)25-19(16)26(15)12-5-4-8-24-10-12/h9,12,24H,4-5,8,10-11H2,1-3H3,(H2,23,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM144614

(US8969586, 1 | US9598436, 1)Show InChI InChI=1S/C19H14N2O/c1-13-10-11-20-12-17(13)15-8-5-9-16-18(21-22-19(15)16)14-6-3-2-4-7-14/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The assays were performed in U-bottom 384-well optiplates. The final assay volume was 15 μl prepared from 7.5 μl additions of microsomes (p... |
US Patent US9598436 (2017)
BindingDB Entry DOI: 10.7270/Q22809N7 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50006830

(CHEMBL3236948)Show SMILES CC(C)n1cc(-c2c(C)n(CC(O)=O)c3ccc(F)cc23)c2ccccc2c1=O |(57.2,-40.02,;55.86,-39.25,;55.86,-37.71,;54.53,-40.02,;54.52,-41.56,;53.19,-42.33,;53.19,-43.87,;54.43,-44.77,;55.89,-44.3,;53.95,-46.24,;54.86,-47.48,;56.39,-47.32,;57.02,-45.92,;57.29,-48.57,;52.41,-46.24,;51.39,-47.37,;49.89,-47.06,;49.41,-45.59,;47.9,-45.27,;50.44,-44.45,;51.94,-44.77,;51.86,-41.56,;50.54,-42.33,;49.21,-41.57,;49.2,-40.03,;50.53,-39.26,;51.86,-40.03,;53.19,-39.25,;53.19,-37.71,)| Show InChI InChI=1S/C23H21FN2O3/c1-13(2)25-11-19(16-6-4-5-7-17(16)23(25)29)22-14(3)26(12-21(27)28)20-9-8-15(24)10-18(20)22/h4-11,13H,12H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CRTH2 receptor-mediated chemotaxis in human basophils |
J Med Chem 57: 1299-322 (2014)
Article DOI: 10.1021/jm401509e
BindingDB Entry DOI: 10.7270/Q21V5GGH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50401097

(CHEMBL2204469)Show SMILES Cc1c(Cc2ccc(=O)n(Cc3ccc(F)cc3F)n2)c2cc(F)ccc2n1CC(O)=O Show InChI InChI=1S/C23H18F3N3O3/c1-13-18(19-8-15(24)4-6-21(19)28(13)12-23(31)32)10-17-5-7-22(30)29(27-17)11-14-2-3-16(25)9-20(14)26/h2-9H,10-12H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CRTH2 receptor-mediated chemotaxis in human basophils |
J Med Chem 55: 5088-109 (2012)
Article DOI: 10.1021/jm300007n
BindingDB Entry DOI: 10.7270/Q2S75HH0 |
More data for this
Ligand-Target Pair | |
Programmed cell death 1 ligand/protein 1
(Homo sapiens-Homo sapiens (Human)) | BDBM50239948
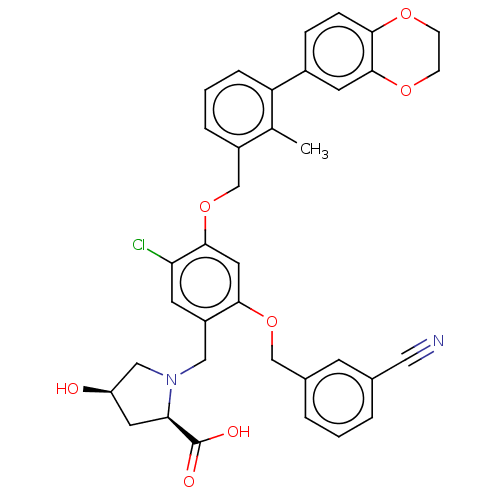
(CHEMBL4071326 | US9850225, Example 1166)Show SMILES Cc1c(COc2cc(OCc3cccc(c3)C#N)c(CN3C[C@H](O)C[C@@H]3C(O)=O)cc2Cl)cccc1-c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C36H33ClN2O7/c1-22-26(6-3-7-29(22)25-8-9-32-35(14-25)44-11-10-43-32)21-46-34-16-33(45-20-24-5-2-4-23(12-24)17-38)27(13-30(34)37)18-39-19-28(40)15-31(39)36(41)42/h2-9,12-14,16,28,31,40H,10-11,15,18-21H2,1H3,(H,41,42)/t28-,31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113998
BindingDB Entry DOI: 10.7270/Q2DR30KP |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50006830

(CHEMBL3236948)Show SMILES CC(C)n1cc(-c2c(C)n(CC(O)=O)c3ccc(F)cc23)c2ccccc2c1=O |(57.2,-40.02,;55.86,-39.25,;55.86,-37.71,;54.53,-40.02,;54.52,-41.56,;53.19,-42.33,;53.19,-43.87,;54.43,-44.77,;55.89,-44.3,;53.95,-46.24,;54.86,-47.48,;56.39,-47.32,;57.02,-45.92,;57.29,-48.57,;52.41,-46.24,;51.39,-47.37,;49.89,-47.06,;49.41,-45.59,;47.9,-45.27,;50.44,-44.45,;51.94,-44.77,;51.86,-41.56,;50.54,-42.33,;49.21,-41.57,;49.2,-40.03,;50.53,-39.26,;51.86,-40.03,;53.19,-39.25,;53.19,-37.71,)| Show InChI InChI=1S/C23H21FN2O3/c1-13(2)25-11-19(16-6-4-5-7-17(16)23(25)29)22-14(3)26(12-21(27)28)20-9-8-15(24)10-18(20)22/h4-11,13H,12H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CRTH2 receptor |
J Med Chem 57: 1299-322 (2014)
Article DOI: 10.1021/jm401509e
BindingDB Entry DOI: 10.7270/Q21V5GGH |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM140958
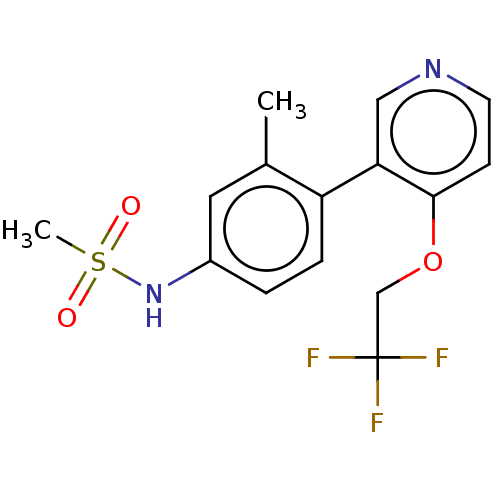
(US8916553, 198)Show InChI InChI=1S/C15H15F3N2O3S/c1-10-7-11(20-24(2,21)22)3-4-12(10)13-8-19-6-5-14(13)23-9-15(16,17)18/h3-8,20H,9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The assays were performed in U-bottom 384-well optiplates. The final assay volume was 15 ul prepared from 7.5 ul additions of microsomes (prepared as... |
US Patent US8916553 (2014)
BindingDB Entry DOI: 10.7270/Q2XP73N9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50518504
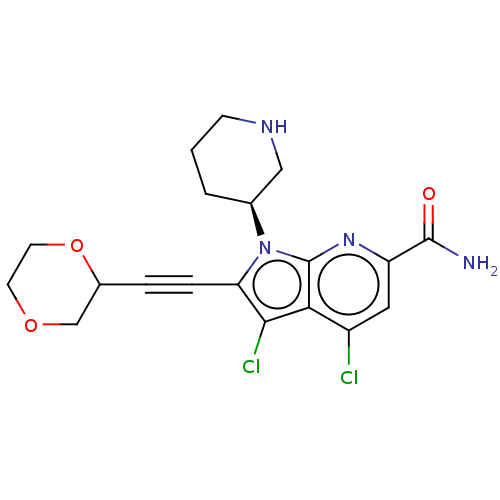
(CHEMBL4466764)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3COCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O3/c20-13-8-14(18(22)26)24-19-16(13)17(21)15(4-3-12-10-27-6-7-28-12)25(19)11-2-1-5-23-9-11/h8,11-12,23H,1-2,5-7,9-10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518513

(CHEMBL4528544)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCOCC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C20H22Cl2N4O2/c21-14-10-15(19(23)27)25-20-17(14)18(22)16(4-3-12-5-8-28-9-6-12)26(20)13-2-1-7-24-11-13/h10,12-13,24H,1-2,5-9,11H2,(H2,23,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Programmed cell death 1 ligand/protein 1
(Homo sapiens-Homo sapiens (Human)) | BDBM50602662
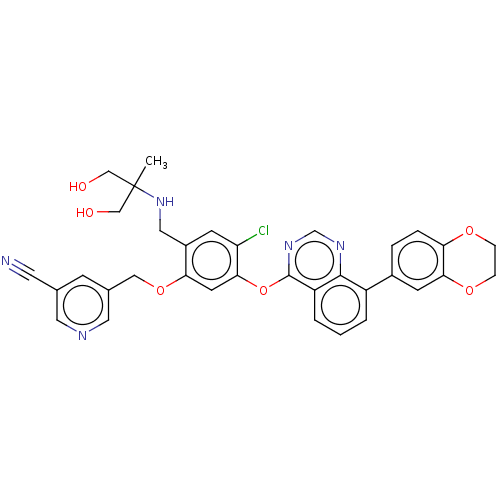
(CHEMBL5201066)Show SMILES CC(CO)(CO)NCc1cc(Cl)c(Oc2ncnc3c(cccc23)-c2ccc3OCCOc3c2)cc1OCc1cncc(c1)C#N | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113998
BindingDB Entry DOI: 10.7270/Q2DR30KP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518511

(CHEMBL4457529)Show SMILES COCC(C)(C)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H24Cl2N4O2/c1-20(2,11-28-3)7-6-15-17(22)16-13(21)9-14(18(23)27)25-19(16)26(15)12-5-4-8-24-10-12/h9,12,24H,4-5,8,10-11H2,1-3H3,(H2,23,27)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data