

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 assessed as reduction in CO2 hydration preincubated for 10 mins by stopped flow assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50276359 (CHEMBL4129303) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate after 15 mins by amplex red reagent based assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM218829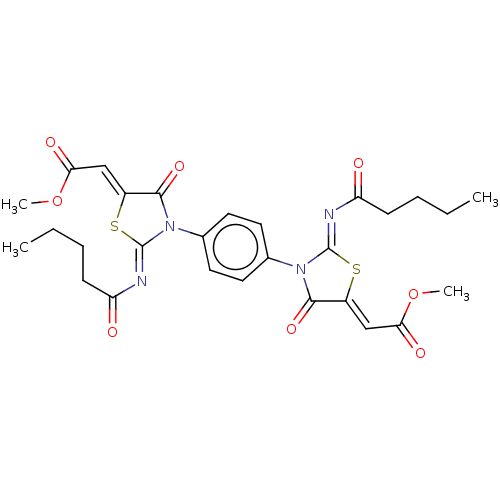 (1,4-Bis(2-pentanoylimino-5-(2-methoxy-2-oxoethylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50044260 (CHEMBL3310053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) | Eur J Med Chem 76: 193-244 (2014) Article DOI: 10.1016/j.ejmech.2014.02.005 BindingDB Entry DOI: 10.7270/Q26T0P8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50276368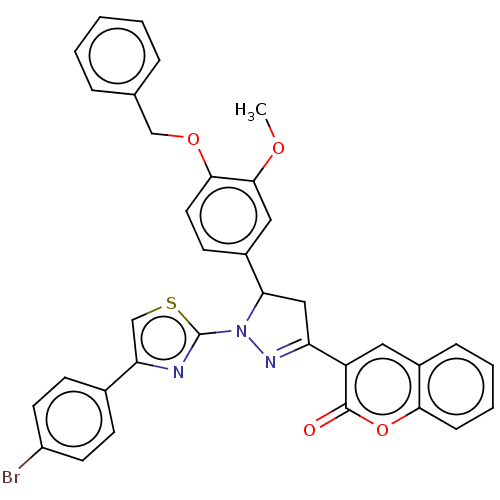 (CHEMBL4128371) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM218826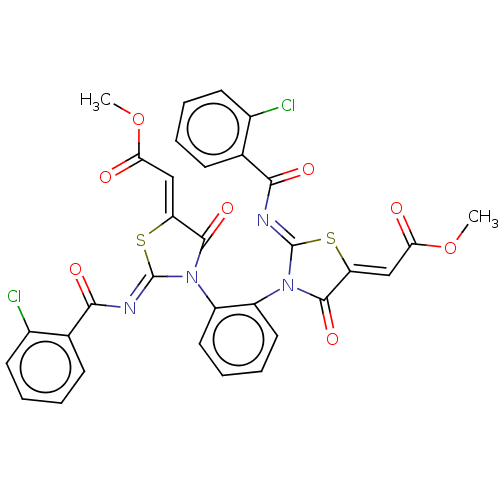 (1,2-Bis(2-(2-chlorobenzoylimino)-5-(2-methoxy-2-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50044259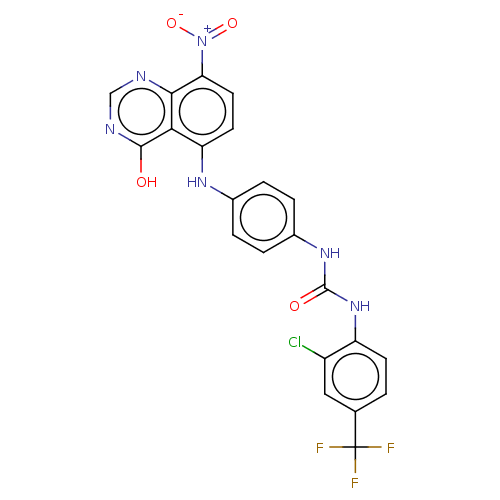 (CHEMBL3310052) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) | Eur J Med Chem 76: 193-244 (2014) Article DOI: 10.1016/j.ejmech.2014.02.005 BindingDB Entry DOI: 10.7270/Q26T0P8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM39862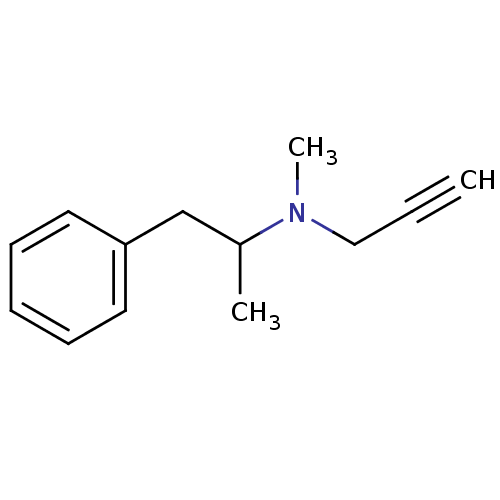 (Deprenyl | METHYL-(1-METHYL-2-PHENYL-ETHYL)-PROP-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE by Ellman's method | Bioorg Med Chem 22: 6163-73 (2014) Article DOI: 10.1016/j.bmc.2014.08.026 BindingDB Entry DOI: 10.7270/Q2JQ12M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human MAO-B using p-tyramine as substrate after 15 mins by amplex red reagent based assay | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50044261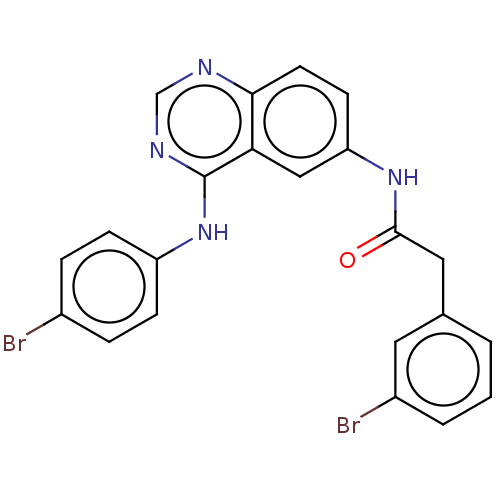 (CHEMBL3310054) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of c-Src (unknown origin) | Eur J Med Chem 76: 193-244 (2014) Article DOI: 10.1016/j.ejmech.2014.02.005 BindingDB Entry DOI: 10.7270/Q26T0P8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 167-77 (2014) Article DOI: 10.1016/j.ejmech.2014.03.046 BindingDB Entry DOI: 10.7270/Q2F76F32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50433370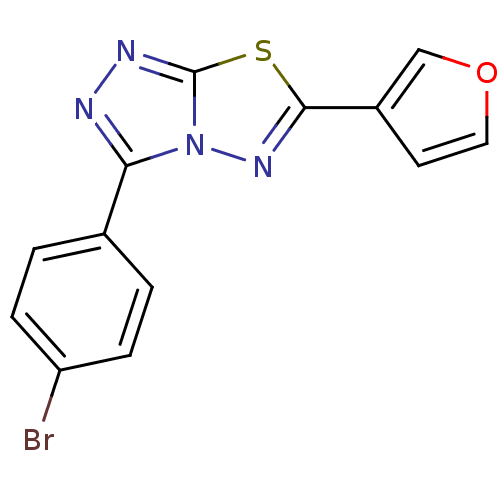 (CHEMBL2375482) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of calf intestinal alkaline phosphatase using p-NPP as substrate incubated for 10 mins prior to substrate addition measured after 30 mins ... | Eur J Med Chem 63: 854-68 (2013) Article DOI: 10.1016/j.ejmech.2013.01.060 BindingDB Entry DOI: 10.7270/Q2222W47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's metho... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448067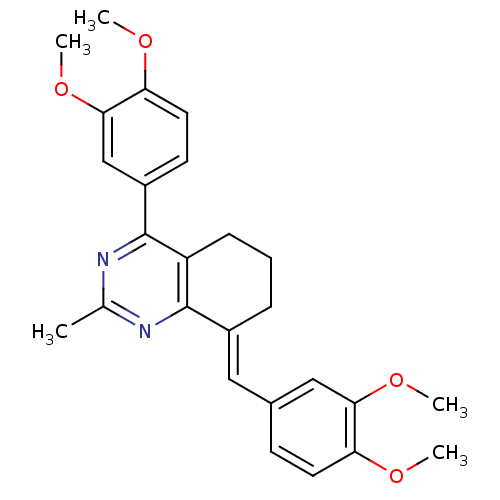 (CHEMBL3115730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of DHFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM193852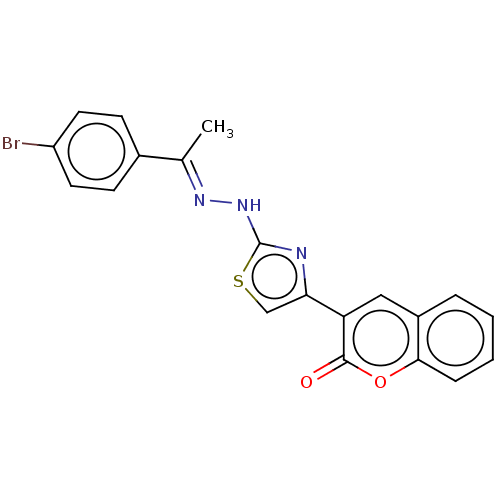 ((E)-3-(2-(2-(1-(4-bromophenyl)ethylidene)hydraziny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description The activity of ALR2 enzyme was determined at 340 nm in UV spectrophotometer that depends upon the measurement of NADPH consumption. Each well of the... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193852 ((E)-3-(2-(2-(1-(4-bromophenyl)ethylidene)hydraziny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM193848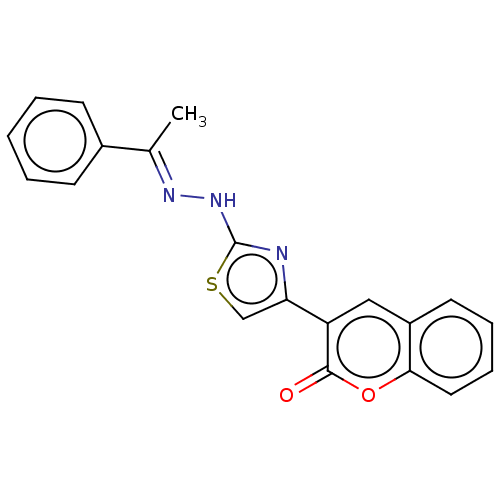 (Coumarin-thiazole series I, 6a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description The activity of ALR2 enzyme was determined at 340 nm in UV spectrophotometer that depends upon the measurement of NADPH consumption. Each well of the... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM193850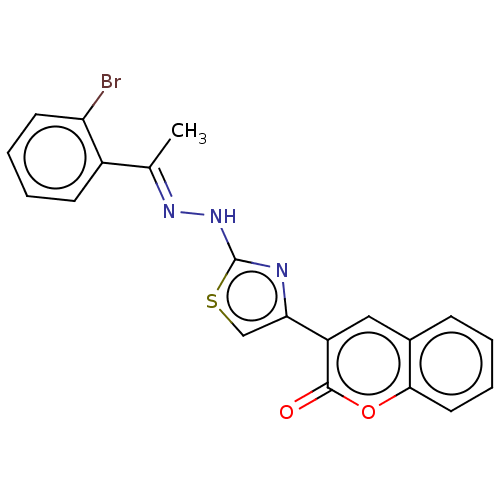 ((E)-3-(2-(2-(1-(2-bromophenyl)ethylidene)hydraziny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description The activity of ALR2 enzyme was determined at 340 nm in UV spectrophotometer that depends upon the measurement of NADPH consumption. Each well of the... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM218824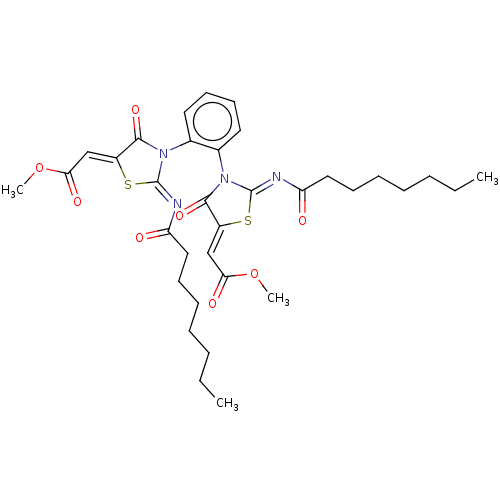 (1,2-Bis(2-octanoylimino-5-(2-methoxy-2-oxoethylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM218823 (1,2-Bis(2-pentanoylimino-5-(2-methoxy-2-oxoethylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX1 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 2 mins by TLC | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50012899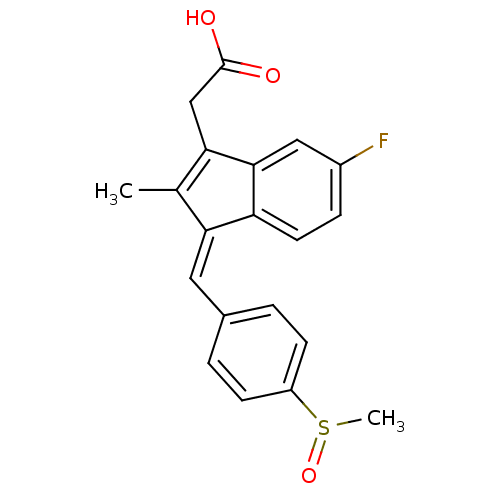 ((Z)-5-Fluoro-2-methyl-1-((p-(methylsulfinyl)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description The activity of ALR2 enzyme was determined at 340 nm in UV spectrophotometer that depends upon the measurement of NADPH consumption. Each well of the... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193866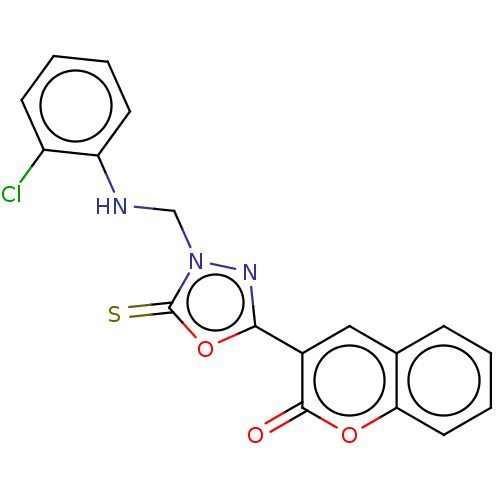 (3-(4-((2-Chlorophenylamino)methyl)-5-thioxo-4,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276310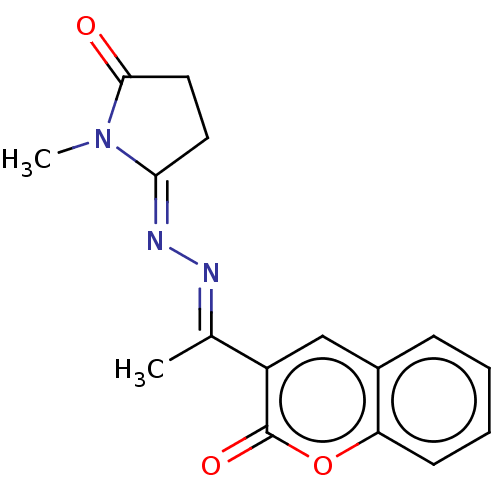 (CHEMBL4127794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM193866 (3-(4-((2-Chlorophenylamino)methyl)-5-thioxo-4,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description The activity of ALR2 enzyme was determined at 340 nm in UV spectrophotometer that depends upon the measurement of NADPH consumption. Each well of the... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276360 (CHEMBL4126559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276366 (CHEMBL4129984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276369 (CHEMBL4127948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM218822 (1,2-Bis(2-butanoylimino-5-(2-methoxy-2-oxoethylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193869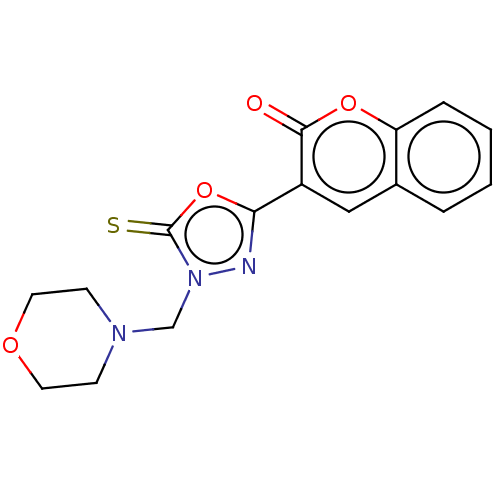 (3-(4-(Morpholinomethyl)-5-thioxo-4,5-dihydro-1,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of calf lens ALR2 using D,L-glyceraldehyde as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV sp... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50331095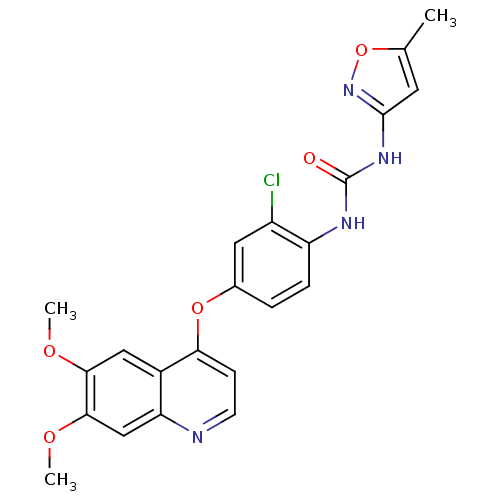 (CHEMBL1289494 | Tivozanib | US10464902, Tivozanib) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) | Eur J Med Chem 76: 193-244 (2014) Article DOI: 10.1016/j.ejmech.2014.02.005 BindingDB Entry DOI: 10.7270/Q26T0P8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM193869 (3-(4-(Morpholinomethyl)-5-thioxo-4,5-dihydro-1,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description The activity of ALR2 enzyme was determined at 340 nm in UV spectrophotometer that depends upon the measurement of NADPH consumption. Each well of the... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50276367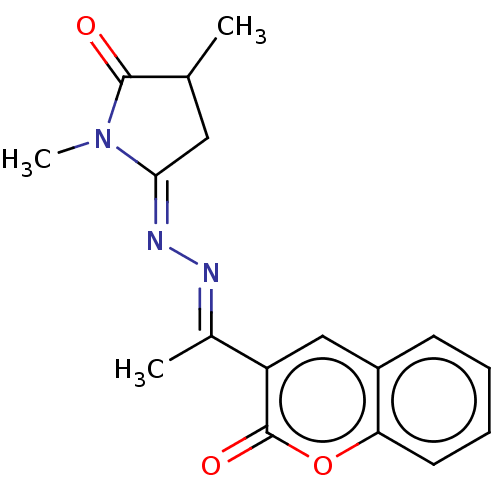 (CHEMBL4129191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human COX2 using [14C]arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 45 mins by TL... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193860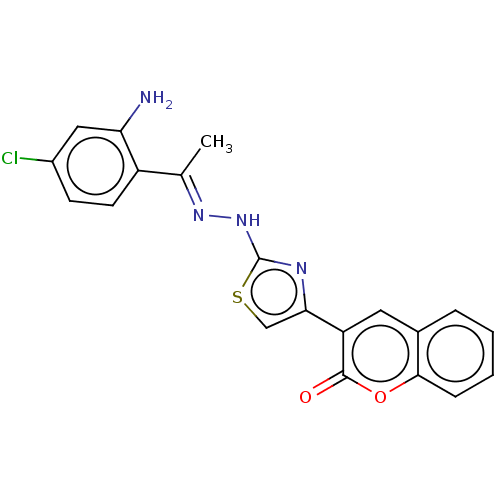 ((E)-3-(2-(2-((2-amino-4-chlorophenyl)(phenyl)methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Bos taurus) | BDBM193860 ((E)-3-(2-(2-((2-amino-4-chlorophenyl)(phenyl)methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of bovine kidney ALR1 using D-glucoronic acid as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by UV... | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50448066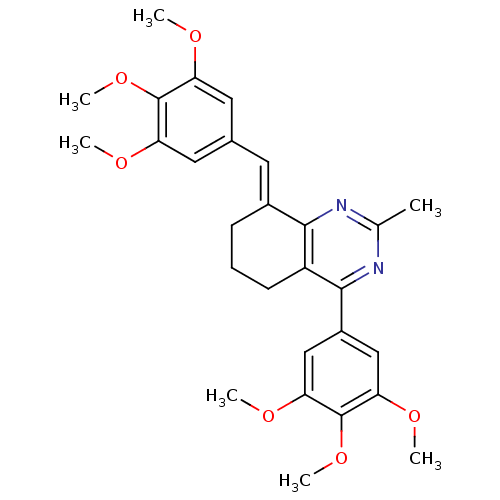 (CHEMBL3115731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of DHFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM218829 (1,4-Bis(2-pentanoylimino-5-(2-methoxy-2-oxoethylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028572 (CHEMBL3338952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE by Ellman's method | Bioorg Med Chem 22: 6163-73 (2014) Article DOI: 10.1016/j.bmc.2014.08.026 BindingDB Entry DOI: 10.7270/Q2JQ12M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM218831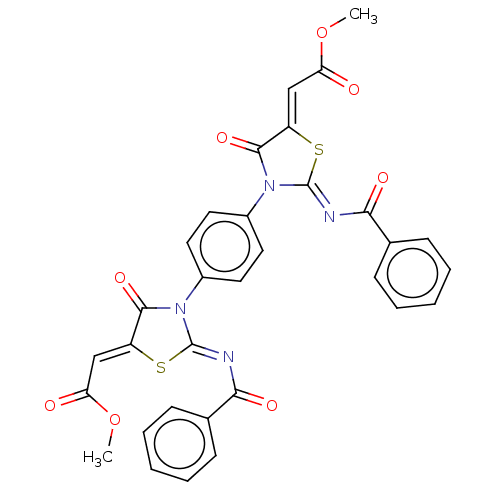 (1,4-Bis(2-benzoylimino-5-(2-methoxy-2-oxoethyliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004319 (CHEMBL3237541) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after ... | Eur J Med Chem 78: 167-77 (2014) Article DOI: 10.1016/j.ejmech.2014.03.046 BindingDB Entry DOI: 10.7270/Q2F76F32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028612 (CHEMBL1466313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 781 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of human BChE by Ellman's method | Bioorg Med Chem 22: 6163-73 (2014) Article DOI: 10.1016/j.bmc.2014.08.026 BindingDB Entry DOI: 10.7270/Q2JQ12M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50004306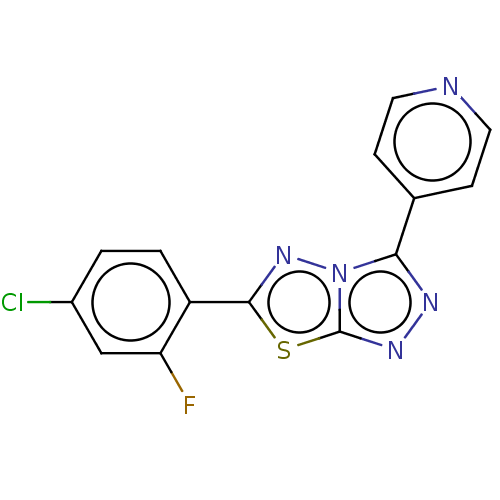 (CHEMBL3237531) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Inhibition of calf intestinal alkaline phosphatase using p-nitrophenyl phosphate as substrate preincubated for 10 mins before substrate addition afte... | Eur J Med Chem 78: 167-77 (2014) Article DOI: 10.1016/j.ejmech.2014.03.046 BindingDB Entry DOI: 10.7270/Q2F76F32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM218824 (1,2-Bis(2-octanoylimino-5-(2-methoxy-2-oxoethylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50062329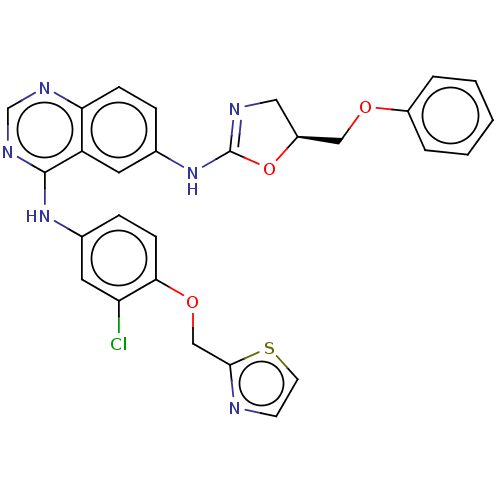 (CHEMBL3397276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Eur J Med Chem 90: 124-69 (2015) Article DOI: 10.1016/j.ejmech.2014.10.084 BindingDB Entry DOI: 10.7270/Q2QZ2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM218823 (1,2-Bis(2-pentanoylimino-5-(2-methoxy-2-oxoethylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Quaid-i-Azam University | Assay Description The assay was performed in a polystyrene 96-well plate having flat bottom containing 200 μL of the total reaction mixture. For each assay, mixtu... | Bioorg Chem 70: 17-26 (2017) Article DOI: 10.1016/j.bioorg.2016.11.004 BindingDB Entry DOI: 10.7270/Q2WM1C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member A1 (Bos taurus (Cattle)) | BDBM193856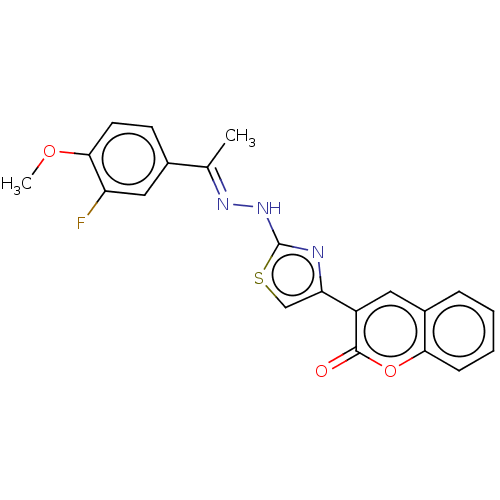 ((E)-3-(2-(2-(1-(3-fluoro-4-methoxyphenyl)ethyliden...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 6.2 | n/a |
COMSATS Institute of Information Technology | Assay Description The assay results were obtained at 340 nm and ALR1 inhibitory activity was measured with the absorbance change at this respective wavelength. Each we... | Bioorg Chem 68: 177-186 (2016) Article DOI: 10.1016/j.bioorg.2016.08.005 BindingDB Entry DOI: 10.7270/Q2445K96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |