 Found 4027 hits with Last Name = 'lyons' and Initial = 'a'
Found 4027 hits with Last Name = 'lyons' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418929
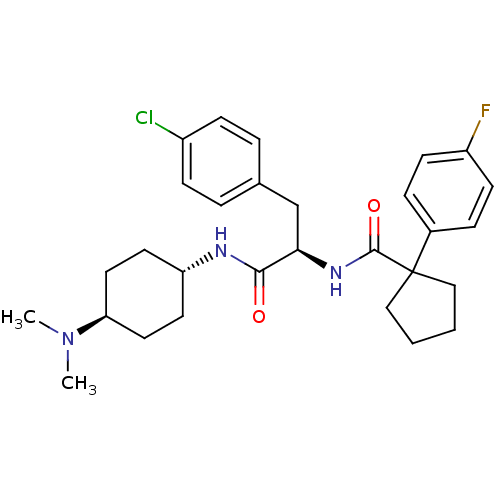
(CHEMBL1807272)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCCC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(27.45,1.1,;26.1,1.87,;26.1,3.43,;24.76,1.09,;23.43,1.85,;22.1,1.08,;22.12,-.45,;23.44,-1.21,;24.76,-.44,;20.8,-1.22,;19.47,-.46,;19.46,1.08,;18.14,-1.23,;18.14,-2.77,;16.81,-3.55,;15.48,-2.78,;14.15,-3.55,;14.16,-5.1,;12.83,-5.87,;15.5,-5.86,;16.83,-5.08,;16.8,-.47,;16.8,1.07,;18.12,1.84,;15.46,1.83,;16.71,2.75,;16.22,4.23,;14.67,4.22,;14.19,2.74,;14.13,1.06,;12.79,1.82,;11.47,1.05,;11.47,-.49,;10.13,-1.26,;12.8,-1.26,;14.13,-.49,)| Show InChI InChI=1S/C29H37ClFN3O2/c1-34(2)25-15-13-24(14-16-25)32-27(35)26(19-20-5-9-22(30)10-6-20)33-28(36)29(17-3-4-18-29)21-7-11-23(31)12-8-21/h5-12,24-26H,3-4,13-19H2,1-2H3,(H,32,35)(H,33,36)/t24-,25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418907

(CHEMBL1807267)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(9.98,-20.03,;8.63,-19.26,;8.62,-17.7,;7.28,-20.04,;5.95,-19.28,;4.62,-20.05,;4.64,-21.58,;5.96,-22.34,;7.28,-21.57,;3.32,-22.35,;1.99,-21.59,;1.98,-20.05,;.65,-22.36,;.66,-23.9,;-.67,-24.68,;-2,-23.91,;-3.33,-24.68,;-3.33,-26.23,;-4.66,-27,;-1.98,-26.99,;-.65,-26.21,;-.68,-21.6,;-.69,-20.06,;.64,-19.28,;-2.03,-19.29,;-1.25,-17.94,;-2.81,-17.95,;-3.35,-20.07,;-4.69,-19.31,;-6.02,-20.08,;-6.02,-21.62,;-7.35,-22.38,;-4.69,-22.39,;-3.35,-21.62,)| Show InChI InChI=1S/C27H33Cl2N3O2/c1-32(2)23-13-11-22(12-14-23)30-25(33)24(17-18-3-7-20(28)8-4-18)31-26(34)27(15-16-27)19-5-9-21(29)10-6-19/h3-10,22-24H,11-17H2,1-2H3,(H,30,33)(H,31,34)/t22-,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418909
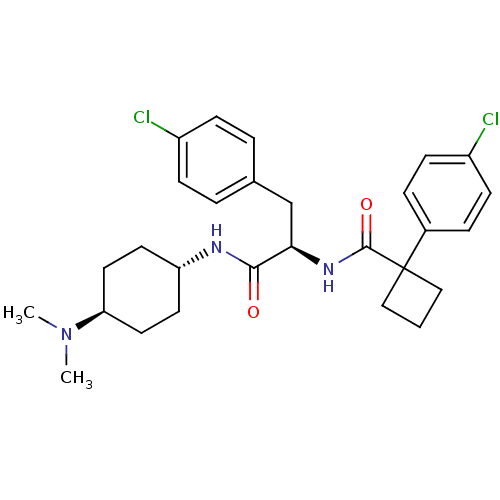
(CHEMBL1807270)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCC1)c1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(30.13,-31,;28.77,-30.23,;28.77,-28.67,;27.43,-31.01,;26.1,-30.25,;24.77,-31.02,;24.79,-32.55,;26.12,-33.31,;27.43,-32.54,;23.48,-33.32,;22.14,-32.56,;22.14,-31.02,;20.81,-33.33,;20.81,-34.87,;19.49,-35.65,;18.15,-34.88,;16.82,-35.65,;16.83,-37.19,;15.5,-37.97,;18.17,-37.96,;19.5,-37.18,;19.47,-32.57,;19.47,-31.03,;20.8,-30.25,;18.13,-30.26,;19.23,-29.16,;18.12,-28.06,;17.02,-29.17,;16.8,-31.04,;15.46,-30.28,;14.14,-31.05,;14.14,-32.59,;12.81,-33.35,;15.47,-33.36,;16.81,-32.59,)| Show InChI InChI=1S/C28H35Cl2N3O2/c1-33(2)24-14-12-23(13-15-24)31-26(34)25(18-19-4-8-21(29)9-5-19)32-27(35)28(16-3-17-28)20-6-10-22(30)11-7-20/h4-11,23-25H,3,12-18H2,1-2H3,(H,31,34)(H,32,35)/t23-,24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418906
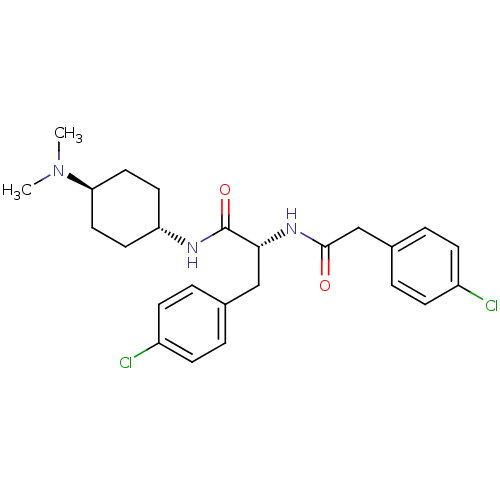
(CHEMBL1807266)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(27.65,-6.84,;26.29,-6.06,;26.29,-4.5,;24.95,-6.85,;23.62,-6.09,;22.29,-6.86,;22.31,-8.38,;23.63,-9.15,;24.95,-8.38,;20.99,-9.16,;19.66,-8.4,;19.65,-6.86,;18.32,-9.17,;18.33,-10.71,;17,-11.49,;15.66,-10.72,;14.33,-11.49,;14.34,-13.03,;13.01,-13.81,;15.68,-13.8,;17.01,-13.02,;16.99,-8.41,;16.98,-6.87,;18.31,-6.09,;15.64,-6.1,;14.31,-6.88,;12.97,-6.11,;11.65,-6.88,;11.64,-8.43,;10.31,-9.19,;12.98,-9.2,;14.32,-8.43,)| Show InChI InChI=1S/C25H31Cl2N3O2/c1-30(2)22-13-11-21(12-14-22)28-25(32)23(15-17-3-7-19(26)8-4-17)29-24(31)16-18-5-9-20(27)10-6-18/h3-10,21-23H,11-16H2,1-2H3,(H,28,32)(H,29,31)/t21-,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418914
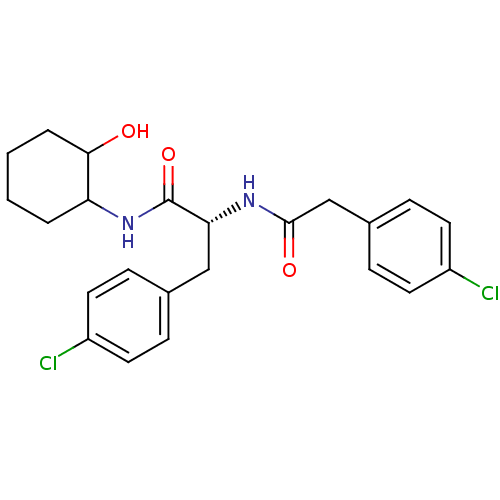
(CHEMBL1807281)Show SMILES OC1CCCCC1NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H26Cl2N2O3/c24-17-9-5-15(6-10-17)13-20(23(30)27-19-3-1-2-4-21(19)28)26-22(29)14-16-7-11-18(25)12-8-16/h5-12,19-21,28H,1-4,13-14H2,(H,26,29)(H,27,30)/t19?,20-,21?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418914

(CHEMBL1807281)Show SMILES OC1CCCCC1NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H26Cl2N2O3/c24-17-9-5-15(6-10-17)13-20(23(30)27-19-3-1-2-4-21(19)28)26-22(29)14-16-7-11-18(25)12-8-16/h5-12,19-21,28H,1-4,13-14H2,(H,26,29)(H,27,30)/t19?,20-,21?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418905
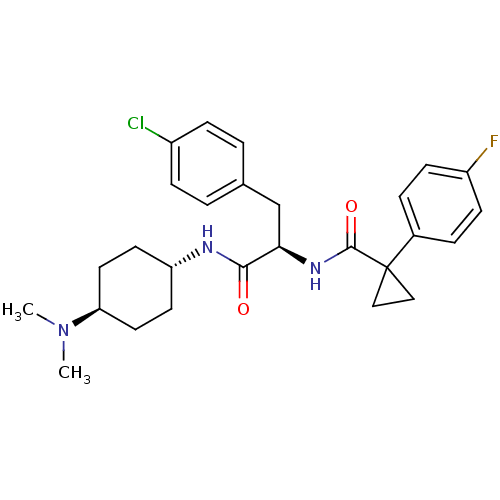
(CHEMBL1807273)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(9.5,-10.04,;8.15,-9.27,;8.14,-7.71,;6.8,-10.05,;5.47,-9.29,;4.14,-10.06,;4.17,-11.59,;5.49,-12.35,;6.8,-11.58,;2.85,-12.36,;1.51,-11.6,;1.51,-10.06,;.18,-12.37,;.18,-13.91,;-1.14,-14.69,;-1.13,-16.22,;-2.46,-17,;-3.8,-16.23,;-5.15,-17.02,;-3.81,-14.69,;-2.48,-13.92,;-1.16,-11.61,;-1.16,-10.07,;.17,-9.29,;-2.5,-9.3,;-1.73,-7.95,;-3.29,-7.96,;-3.83,-10.08,;-5.17,-9.31,;-6.49,-10.09,;-6.5,-11.63,;-7.83,-12.39,;-5.17,-12.4,;-3.83,-11.63,)| Show InChI InChI=1S/C27H33ClFN3O2/c1-32(2)23-13-11-22(12-14-23)30-25(33)24(17-18-3-7-20(28)8-4-18)31-26(34)27(15-16-27)19-5-9-21(29)10-6-19/h3-10,22-24H,11-17H2,1-2H3,(H,30,33)(H,31,34)/t22-,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418910
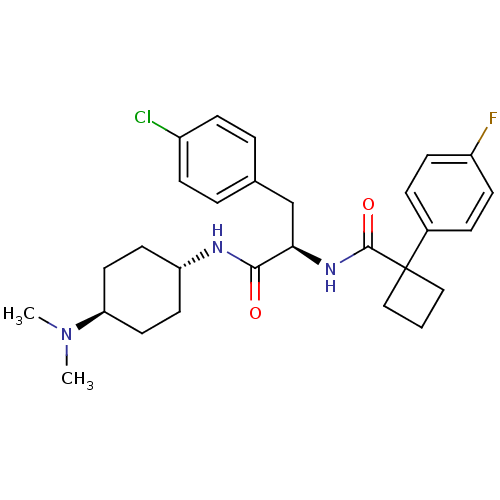
(CHEMBL1807271)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(9.02,1.23,;7.67,2.01,;7.66,3.57,;6.32,1.22,;4.99,1.98,;3.67,1.21,;3.69,-.31,;5.01,-1.07,;6.33,-.31,;2.37,-1.09,;1.04,-.32,;1.03,1.21,;-.3,-1.1,;-.29,-2.64,;-1.62,-3.41,;-2.95,-2.64,;-4.28,-3.42,;-4.27,-4.96,;-5.61,-5.73,;-2.93,-5.72,;-1.6,-4.94,;-1.63,-.33,;-1.64,1.2,;-.31,1.98,;-2.97,1.97,;-1.88,3.08,;-2.98,4.18,;-4.08,3.07,;-4.3,1.19,;-5.64,1.96,;-6.97,1.19,;-6.97,-.35,;-8.3,-1.12,;-5.64,-1.12,;-4.3,-.35,)| Show InChI InChI=1S/C28H35ClFN3O2/c1-33(2)24-14-12-23(13-15-24)31-26(34)25(18-19-4-8-21(29)9-5-19)32-27(35)28(16-3-17-28)20-6-10-22(30)11-7-20/h4-11,23-25H,3,12-18H2,1-2H3,(H,31,34)(H,32,35)/t23-,24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418930

(CHEMBL1807278)Show SMILES CC(CCO)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H24Cl2N2O3/c1-14(10-11-26)24-21(28)19(12-15-2-6-17(22)7-3-15)25-20(27)13-16-4-8-18(23)9-5-16/h2-9,14,19,26H,10-13H2,1H3,(H,24,28)(H,25,27)/t14?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418930

(CHEMBL1807278)Show SMILES CC(CCO)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H24Cl2N2O3/c1-14(10-11-26)24-21(28)19(12-15-2-6-17(22)7-3-15)25-20(27)13-16-4-8-18(23)9-5-16/h2-9,14,19,26H,10-13H2,1H3,(H,24,28)(H,25,27)/t14?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418908
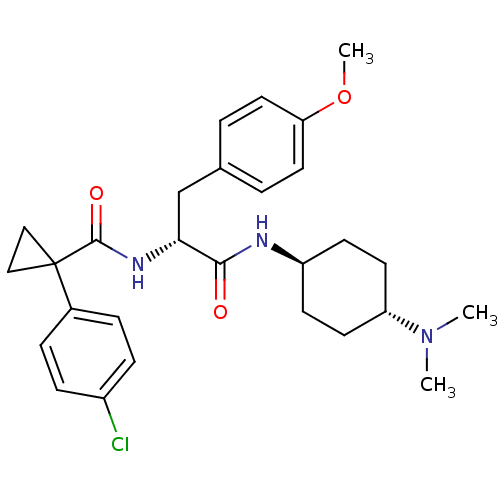
(CHEMBL1807268)Show SMILES COc1ccc(C[C@@H](NC(=O)C2(CC2)c2ccc(Cl)cc2)C(=O)N[C@H]2CC[C@@H](CC2)N(C)C)cc1 |r,wU:24.25,7.7,wD:27.32,(14.38,-24.59,;15.73,-25.36,;17.08,-24.58,;17.07,-23.04,;18.4,-22.26,;19.73,-23.03,;21.06,-22.26,;21.06,-20.72,;19.72,-19.95,;19.72,-18.41,;21.04,-17.64,;18.38,-17.65,;19.15,-16.29,;17.59,-16.3,;17.05,-18.42,;15.71,-17.66,;14.38,-18.43,;14.38,-19.97,;13.05,-20.74,;15.71,-20.74,;17.05,-19.97,;22.39,-19.94,;22.38,-18.4,;23.72,-20.71,;25.04,-19.93,;25.02,-18.4,;26.35,-17.63,;27.68,-18.39,;27.68,-19.92,;26.37,-20.69,;29.03,-17.61,;30.38,-18.38,;29.02,-16.05,;19.75,-24.57,;18.42,-25.35,)| Show InChI InChI=1S/C28H36ClN3O3/c1-32(2)23-12-10-22(11-13-23)30-26(33)25(18-19-4-14-24(35-3)15-5-19)31-27(34)28(16-17-28)20-6-8-21(29)9-7-20/h4-9,14-15,22-23,25H,10-13,16-18H2,1-3H3,(H,30,33)(H,31,34)/t22-,23-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418928
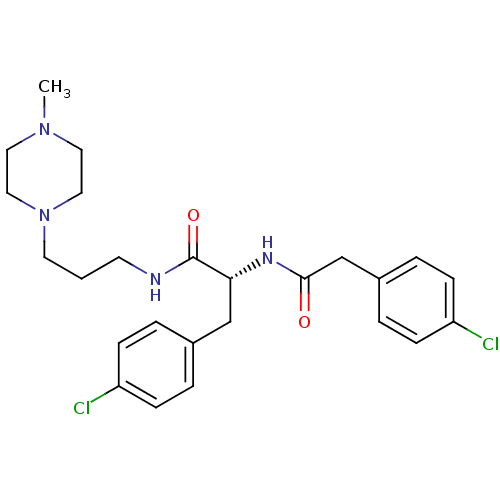
(CHEMBL1807259)Show SMILES CN1CCN(CCCNC(=O)[C@@H](Cc2ccc(Cl)cc2)NC(=O)Cc2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C25H32Cl2N4O2/c1-30-13-15-31(16-14-30)12-2-11-28-25(33)23(17-19-3-7-21(26)8-4-19)29-24(32)18-20-5-9-22(27)10-6-20/h3-10,23H,2,11-18H2,1H3,(H,28,33)(H,29,32)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418924
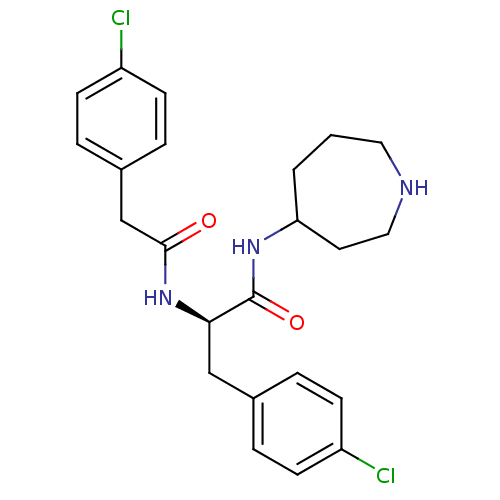
(CHEMBL1807256)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)NC2CCCNCC2)cc1 |r| Show InChI InChI=1S/C23H27Cl2N3O2/c24-18-7-3-16(4-8-18)14-21(23(30)27-20-2-1-12-26-13-11-20)28-22(29)15-17-5-9-19(25)10-6-17/h3-10,20-21,26H,1-2,11-15H2,(H,27,30)(H,28,29)/t20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50358567
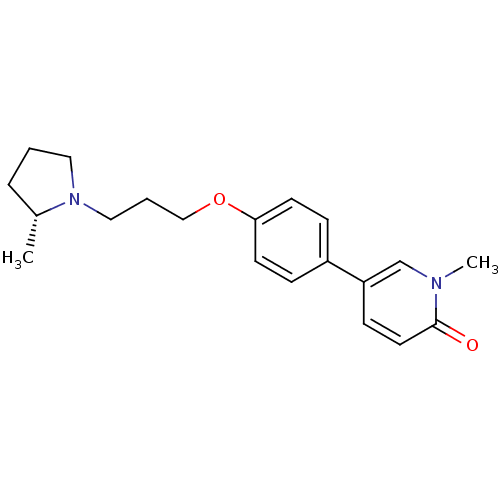
(CHEMBL1923729)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(C)c1 |r| Show InChI InChI=1S/C20H26N2O2/c1-16-5-3-12-22(16)13-4-14-24-19-9-6-17(7-10-19)18-8-11-20(23)21(2)15-18/h6-11,15-16H,3-5,12-14H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50358577
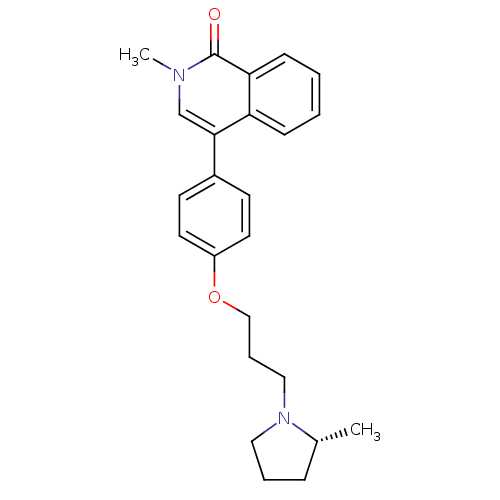
(CHEMBL1923738)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cn(C)c(=O)c2ccccc12 |r| Show InChI InChI=1S/C24H28N2O2/c1-18-7-5-14-26(18)15-6-16-28-20-12-10-19(11-13-20)23-17-25(2)24(27)22-9-4-3-8-21(22)23/h3-4,8-13,17-18H,5-7,14-16H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418911
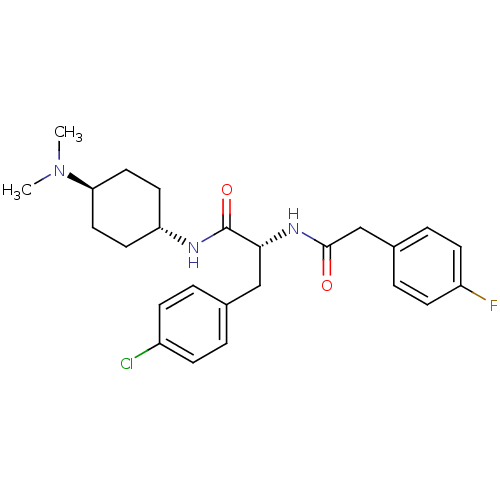
(CHEMBL1807274)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(27.69,-10,;26.33,-9.22,;26.33,-7.67,;24.98,-10.01,;23.65,-9.25,;22.32,-10.02,;22.35,-11.55,;23.67,-12.31,;24.99,-11.54,;21.03,-12.32,;19.69,-11.56,;19.69,-10.02,;18.36,-12.33,;18.36,-13.87,;17.04,-14.65,;15.7,-13.88,;14.37,-14.65,;14.38,-16.2,;13.03,-16.98,;15.72,-16.97,;17.05,-16.18,;17.02,-11.57,;17.02,-10.03,;18.35,-9.25,;15.68,-9.26,;14.35,-10.04,;13.01,-9.27,;11.68,-10.04,;11.68,-11.59,;10.35,-12.35,;13.01,-12.36,;14.35,-11.59,)| Show InChI InChI=1S/C25H31ClFN3O2/c1-30(2)22-13-11-21(12-14-22)28-25(32)23(15-17-3-7-19(26)8-4-17)29-24(31)16-18-5-9-20(27)10-6-18/h3-10,21-23H,11-16H2,1-2H3,(H,28,32)(H,29,31)/t21-,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418929

(CHEMBL1807272)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCCC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(27.45,1.1,;26.1,1.87,;26.1,3.43,;24.76,1.09,;23.43,1.85,;22.1,1.08,;22.12,-.45,;23.44,-1.21,;24.76,-.44,;20.8,-1.22,;19.47,-.46,;19.46,1.08,;18.14,-1.23,;18.14,-2.77,;16.81,-3.55,;15.48,-2.78,;14.15,-3.55,;14.16,-5.1,;12.83,-5.87,;15.5,-5.86,;16.83,-5.08,;16.8,-.47,;16.8,1.07,;18.12,1.84,;15.46,1.83,;16.71,2.75,;16.22,4.23,;14.67,4.22,;14.19,2.74,;14.13,1.06,;12.79,1.82,;11.47,1.05,;11.47,-.49,;10.13,-1.26,;12.8,-1.26,;14.13,-.49,)| Show InChI InChI=1S/C29H37ClFN3O2/c1-34(2)25-15-13-24(14-16-25)32-27(35)26(19-20-5-9-22(30)10-6-20)33-28(36)29(17-3-4-18-29)21-7-11-23(31)12-8-21/h5-12,24-26H,3-4,13-19H2,1-2H3,(H,32,35)(H,33,36)/t24-,25-,26-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418907

(CHEMBL1807267)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(9.98,-20.03,;8.63,-19.26,;8.62,-17.7,;7.28,-20.04,;5.95,-19.28,;4.62,-20.05,;4.64,-21.58,;5.96,-22.34,;7.28,-21.57,;3.32,-22.35,;1.99,-21.59,;1.98,-20.05,;.65,-22.36,;.66,-23.9,;-.67,-24.68,;-2,-23.91,;-3.33,-24.68,;-3.33,-26.23,;-4.66,-27,;-1.98,-26.99,;-.65,-26.21,;-.68,-21.6,;-.69,-20.06,;.64,-19.28,;-2.03,-19.29,;-1.25,-17.94,;-2.81,-17.95,;-3.35,-20.07,;-4.69,-19.31,;-6.02,-20.08,;-6.02,-21.62,;-7.35,-22.38,;-4.69,-22.39,;-3.35,-21.62,)| Show InChI InChI=1S/C27H33Cl2N3O2/c1-32(2)23-13-11-22(12-14-23)30-25(33)24(17-18-3-7-20(28)8-4-18)31-26(34)27(15-16-27)19-5-9-21(29)10-6-19/h3-10,22-24H,11-17H2,1-2H3,(H,30,33)(H,31,34)/t22-,23-,24-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418923
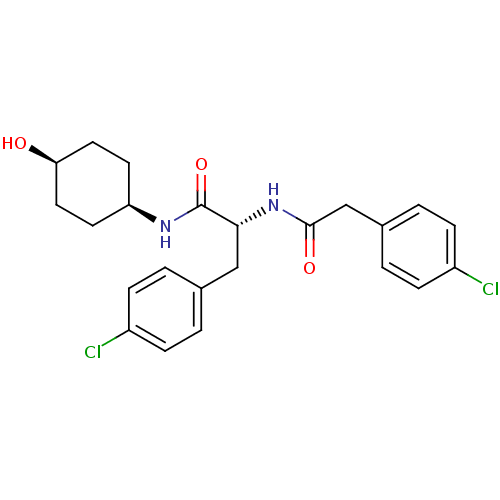
(CHEMBL1807280)Show SMILES O[C@H]1CC[C@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r,wU:10.20,wD:4.7,1.0,(26.16,4.03,;24.81,3.24,;23.48,4,;22.15,3.23,;22.17,1.71,;23.49,.94,;24.81,1.71,;20.85,.94,;19.51,1.7,;19.5,3.24,;18.18,.93,;18.18,-.62,;16.85,-1.39,;15.52,-.62,;14.19,-1.4,;14.19,-2.94,;12.86,-3.71,;15.54,-3.71,;16.87,-2.93,;16.84,1.69,;16.84,3.23,;18.16,4.01,;15.5,4,;14.17,3.22,;12.83,3.98,;11.5,3.21,;11.5,1.67,;10.17,.9,;12.83,.9,;14.17,1.67,)| Show InChI InChI=1S/C23H26Cl2N2O3/c24-17-5-1-15(2-6-17)13-21(23(30)26-19-9-11-20(28)12-10-19)27-22(29)14-16-3-7-18(25)8-4-16/h1-8,19-21,28H,9-14H2,(H,26,30)(H,27,29)/t19-,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50358572
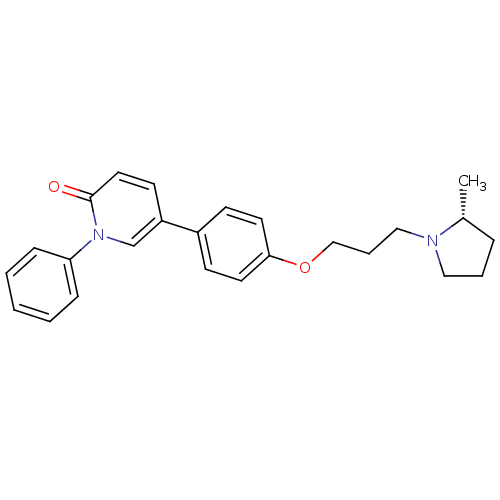
(CHEMBL1923733)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(c1)-c1ccccc1 |r| Show InChI InChI=1S/C25H28N2O2/c1-20-7-5-16-26(20)17-6-18-29-24-13-10-21(11-14-24)22-12-15-25(28)27(19-22)23-8-3-2-4-9-23/h2-4,8-15,19-20H,5-7,16-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50364972
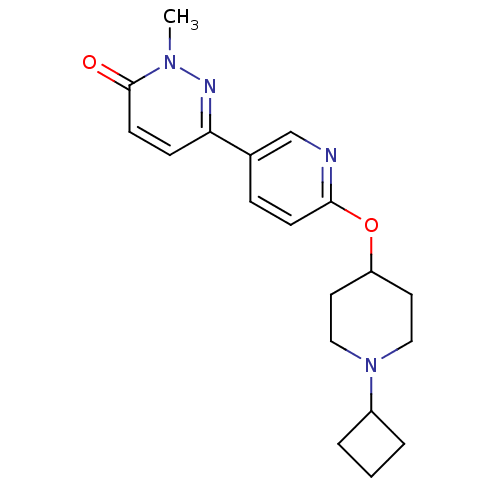
(CHEMBL1950738)Show InChI InChI=1S/C19H24N4O2/c1-22-19(24)8-6-17(21-22)14-5-7-18(20-13-14)25-16-9-11-23(12-10-16)15-3-2-4-15/h5-8,13,15-16H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50364957
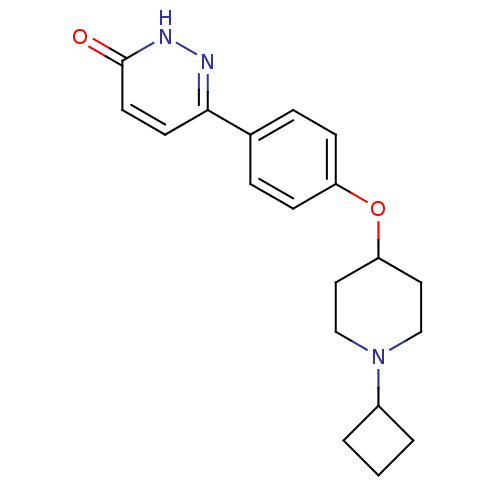
(CHEMBL1950643)Show SMILES O=c1ccc(n[nH]1)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C19H23N3O2/c23-19-9-8-18(20-21-19)14-4-6-16(7-5-14)24-17-10-12-22(13-11-17)15-2-1-3-15/h4-9,15,17H,1-3,10-13H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360899
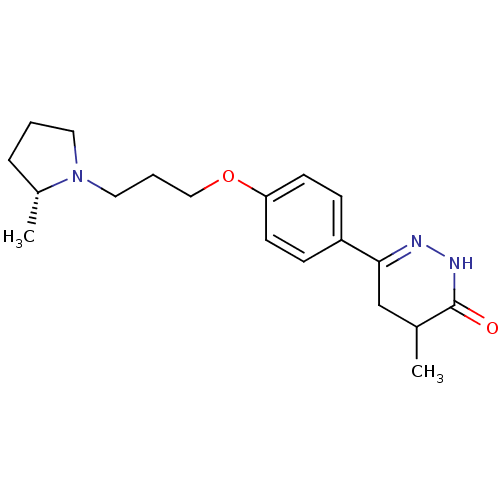
(CHEMBL1935110)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)C(C)C1 |r,t:18| Show InChI InChI=1S/C19H27N3O2/c1-14-13-18(20-21-19(14)23)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,14-15H,3-5,10-13H2,1-2H3,(H,21,23)/t14?,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350021
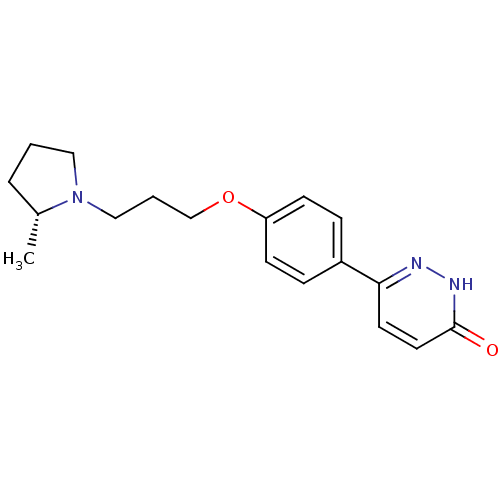
(CHEMBL1813067)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)[nH]n1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-10,14H,2-4,11-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Global R&D.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Eur J Med Chem 95: 349-56 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.054
BindingDB Entry DOI: 10.7270/Q2C24Z4M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50379619
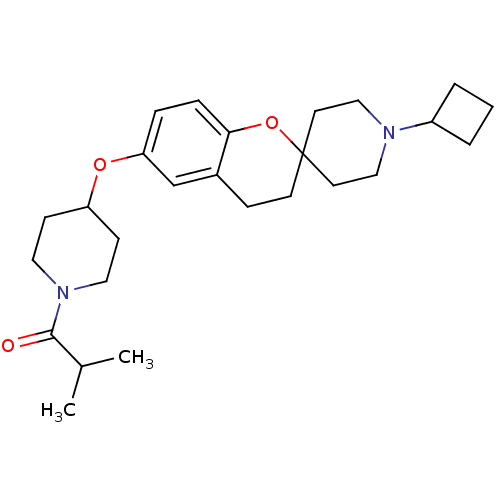
(CHEMBL2013052)Show SMILES CC(C)C(=O)N1CCC(CC1)Oc1ccc2OC3(CCN(CC3)C3CCC3)CCc2c1 Show InChI InChI=1S/C26H38N2O3/c1-19(2)25(29)28-14-9-22(10-15-28)30-23-6-7-24-20(18-23)8-11-26(31-24)12-16-27(17-13-26)21-4-3-5-21/h6-7,18-19,21-22H,3-5,8-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2151-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.139
BindingDB Entry DOI: 10.7270/Q2736RX9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360897
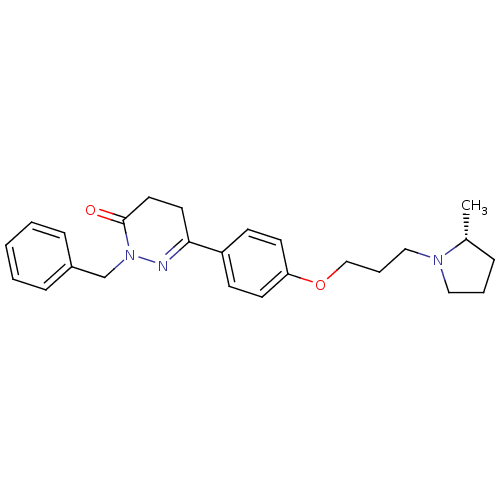
(CHEMBL1935108)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NN(Cc2ccccc2)C(=O)CC1 |r,t:18| Show InChI InChI=1S/C25H31N3O2/c1-20-7-5-16-27(20)17-6-18-30-23-12-10-22(11-13-23)24-14-15-25(29)28(26-24)19-21-8-3-2-4-9-21/h2-4,8-13,20H,5-7,14-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50358574
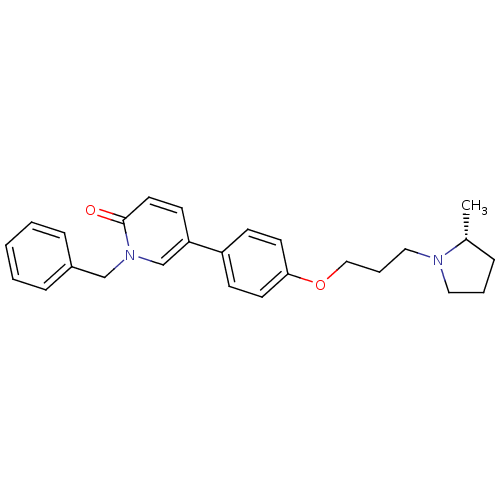
(CHEMBL1923735)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccc(=O)n(Cc2ccccc2)c1 |r| Show InChI InChI=1S/C26H30N2O2/c1-21-7-5-16-27(21)17-6-18-30-25-13-10-23(11-14-25)24-12-15-26(29)28(20-24)19-22-8-3-2-4-9-22/h2-4,8-15,20-21H,5-7,16-19H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418909

(CHEMBL1807270)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CCC1)c1ccc(Cl)cc1 |r,wU:6.9,12.22,wD:3.2,(30.13,-31,;28.77,-30.23,;28.77,-28.67,;27.43,-31.01,;26.1,-30.25,;24.77,-31.02,;24.79,-32.55,;26.12,-33.31,;27.43,-32.54,;23.48,-33.32,;22.14,-32.56,;22.14,-31.02,;20.81,-33.33,;20.81,-34.87,;19.49,-35.65,;18.15,-34.88,;16.82,-35.65,;16.83,-37.19,;15.5,-37.97,;18.17,-37.96,;19.5,-37.18,;19.47,-32.57,;19.47,-31.03,;20.8,-30.25,;18.13,-30.26,;19.23,-29.16,;18.12,-28.06,;17.02,-29.17,;16.8,-31.04,;15.46,-30.28,;14.14,-31.05,;14.14,-32.59,;12.81,-33.35,;15.47,-33.36,;16.81,-32.59,)| Show InChI InChI=1S/C28H35Cl2N3O2/c1-33(2)24-14-12-23(13-15-24)31-26(34)25(18-19-4-8-21(29)9-5-19)32-27(35)28(16-3-17-28)20-6-10-22(30)11-7-20/h4-11,23-25H,3,12-18H2,1-2H3,(H,31,34)(H,32,35)/t23-,24-,25-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50364957

(CHEMBL1950643)Show SMILES O=c1ccc(n[nH]1)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C19H23N3O2/c23-19-9-8-18(20-21-19)14-4-6-16(7-5-14)24-17-10-12-22(13-11-17)15-2-1-3-15/h4-9,15,17H,1-3,10-13H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418905

(CHEMBL1807273)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(F)cc1 |r,wU:6.9,12.22,wD:3.2,(9.5,-10.04,;8.15,-9.27,;8.14,-7.71,;6.8,-10.05,;5.47,-9.29,;4.14,-10.06,;4.17,-11.59,;5.49,-12.35,;6.8,-11.58,;2.85,-12.36,;1.51,-11.6,;1.51,-10.06,;.18,-12.37,;.18,-13.91,;-1.14,-14.69,;-1.13,-16.22,;-2.46,-17,;-3.8,-16.23,;-5.15,-17.02,;-3.81,-14.69,;-2.48,-13.92,;-1.16,-11.61,;-1.16,-10.07,;.17,-9.29,;-2.5,-9.3,;-1.73,-7.95,;-3.29,-7.96,;-3.83,-10.08,;-5.17,-9.31,;-6.49,-10.09,;-6.5,-11.63,;-7.83,-12.39,;-5.17,-12.4,;-3.83,-11.63,)| Show InChI InChI=1S/C27H33ClFN3O2/c1-32(2)23-13-11-22(12-14-23)30-25(33)24(17-18-3-7-20(28)8-4-18)31-26(34)27(15-16-27)19-5-9-21(29)10-6-19/h3-10,22-24H,11-17H2,1-2H3,(H,30,33)(H,31,34)/t22-,23-,24-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50079579

(CHEMBL3417505)Show SMILES C[C@H](COc1ccc(cc1)C1=NNC(=O)C2CC12)CN1CCC[C@H]1C |r,t:11| Show InChI InChI=1S/C20H27N3O2/c1-13(11-23-9-3-4-14(23)2)12-25-16-7-5-15(6-8-16)19-17-10-18(17)20(24)22-21-19/h5-8,13-14,17-18H,3-4,9-12H2,1-2H3,(H,22,24)/t13-,14+,17?,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Global R&D.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Eur J Med Chem 95: 349-56 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.054
BindingDB Entry DOI: 10.7270/Q2C24Z4M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50352798
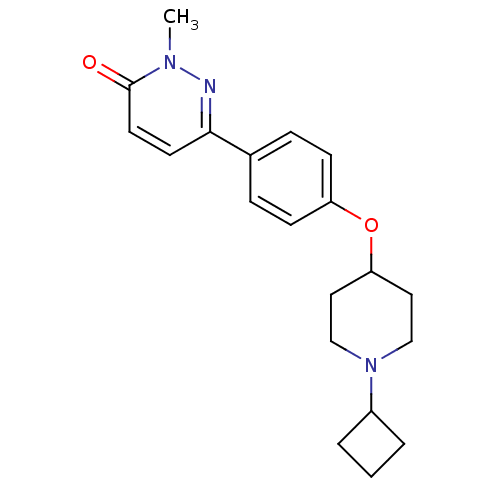
(CHEMBL1823402)Show InChI InChI=1S/C20H25N3O2/c1-22-20(24)10-9-19(21-22)15-5-7-17(8-6-15)25-18-11-13-23(14-12-18)16-3-2-4-16/h5-10,16,18H,2-4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50358577

(CHEMBL1923738)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cn(C)c(=O)c2ccccc12 |r| Show InChI InChI=1S/C24H28N2O2/c1-18-7-5-14-26(18)15-6-16-28-20-12-10-19(11-13-20)23-17-25(2)24(27)22-9-4-3-8-21(22)23/h3-4,8-13,17-18H,5-7,14-16H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50417843
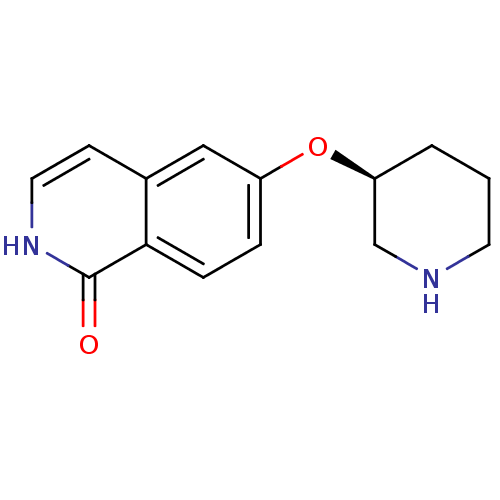
(CHEMBL1667967)Show InChI InChI=1S/C14H16N2O2/c17-14-13-4-3-11(8-10(13)5-7-16-14)18-12-2-1-6-15-9-12/h3-5,7-8,12,15H,1-2,6,9H2,(H,16,17)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Binding affinity to ROCK1 |
Bioorg Med Chem Lett 21: 1084-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.104
BindingDB Entry DOI: 10.7270/Q2DN468G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50364958
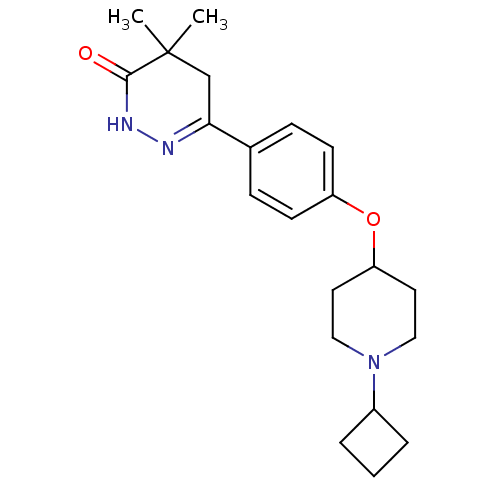
(CHEMBL1950743)Show SMILES CC1(C)CC(=NNC1=O)c1ccc(OC2CCN(CC2)C2CCC2)cc1 |c:4| Show InChI InChI=1S/C21H29N3O2/c1-21(2)14-19(22-23-20(21)25)15-6-8-17(9-7-15)26-18-10-12-24(13-11-18)16-4-3-5-16/h6-9,16,18H,3-5,10-14H2,1-2H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50352799
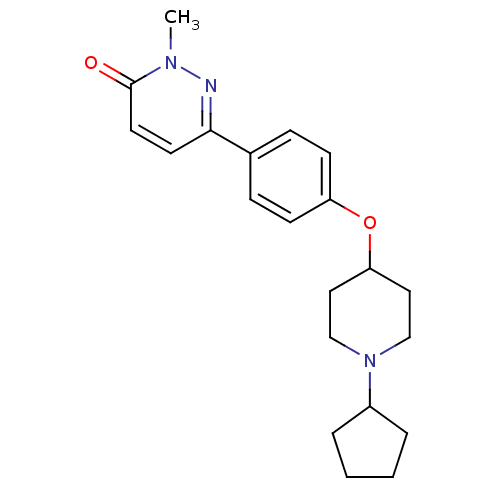
(CHEMBL1823403)Show InChI InChI=1S/C21H27N3O2/c1-23-21(25)11-10-20(22-23)16-6-8-18(9-7-16)26-19-12-14-24(15-13-19)17-4-2-3-5-17/h6-11,17,19H,2-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418912
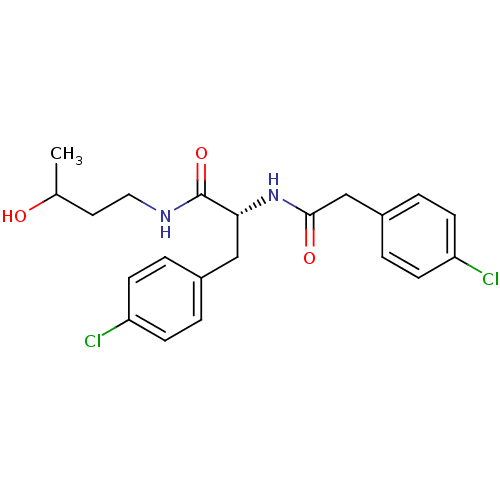
(CHEMBL1807277)Show SMILES CC(O)CCNC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H24Cl2N2O3/c1-14(26)10-11-24-21(28)19(12-15-2-6-17(22)7-3-15)25-20(27)13-16-4-8-18(23)9-5-16/h2-9,14,19,26H,10-13H2,1H3,(H,24,28)(H,25,27)/t14?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418912

(CHEMBL1807277)Show SMILES CC(O)CCNC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H24Cl2N2O3/c1-14(26)10-11-24-21(28)19(12-15-2-6-17(22)7-3-15)25-20(27)13-16-4-8-18(23)9-5-16/h2-9,14,19,26H,10-13H2,1H3,(H,24,28)(H,25,27)/t14?,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50353167
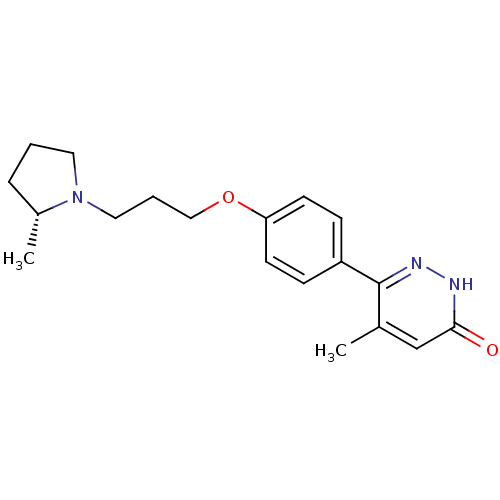
(CHEMBL1829475)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1n[nH]c(=O)cc1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14-13-18(23)20-21-19(14)16-6-8-17(9-7-16)24-12-4-11-22-10-3-5-15(22)2/h6-9,13,15H,3-5,10-12H2,1-2H3,(H,20,23)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50385246
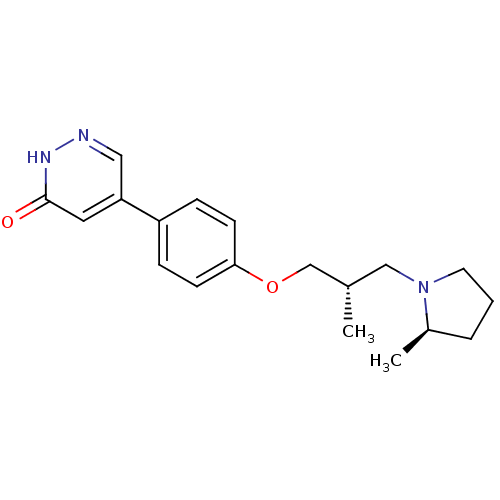
(CHEMBL2037604)Show SMILES C[C@H](COc1ccc(cc1)-c1cn[nH]c(=O)c1)CN1CCC[C@H]1C |r| Show InChI InChI=1S/C19H25N3O2/c1-14(12-22-9-3-4-15(22)2)13-24-18-7-5-16(6-8-18)17-10-19(23)21-20-11-17/h5-8,10-11,14-15H,3-4,9,12-13H2,1-2H3,(H,21,23)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in CHO cells after 4 hrs by scintillation proximity assay |
Bioorg Med Chem 20: 3880-6 (2012)
Article DOI: 10.1016/j.bmc.2012.04.028
BindingDB Entry DOI: 10.7270/Q27D2W64 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50350023
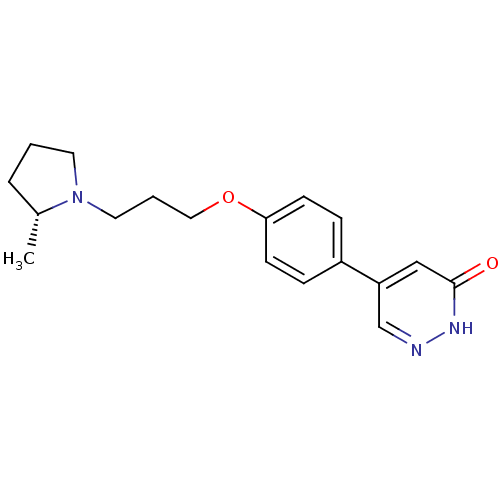
(CHEMBL1813065)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cn[nH]c(=O)c1 |r| Show InChI InChI=1S/C18H23N3O2/c1-14-4-2-9-21(14)10-3-11-23-17-7-5-15(6-8-17)16-12-18(22)20-19-13-16/h5-8,12-14H,2-4,9-11H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nalpha-methylhistamine from human histamine H3 receptor expressed in CHO cells after 4 hrs by scintillation proximity assay |
Bioorg Med Chem 20: 3880-6 (2012)
Article DOI: 10.1016/j.bmc.2012.04.028
BindingDB Entry DOI: 10.7270/Q27D2W64 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50379614
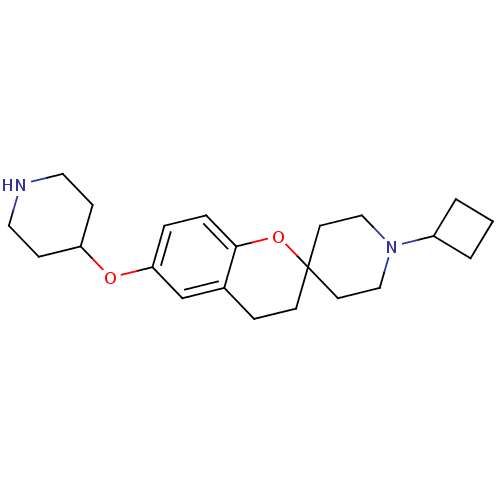
(CHEMBL2013048)Show InChI InChI=1S/C22H32N2O2/c1-2-18(3-1)24-14-10-22(11-15-24)9-6-17-16-20(4-5-21(17)26-22)25-19-7-12-23-13-8-19/h4-5,16,18-19,23H,1-3,6-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 2151-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.139
BindingDB Entry DOI: 10.7270/Q2736RX9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50358575
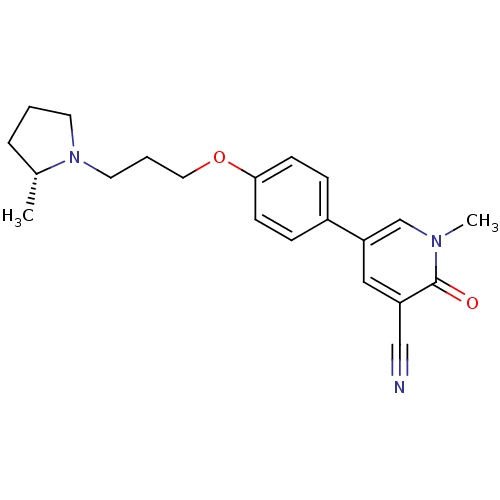
(CHEMBL1923736)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(C#N)c(=O)n(C)c1 |r| Show InChI InChI=1S/C21H25N3O2/c1-16-5-3-10-24(16)11-4-12-26-20-8-6-17(7-9-20)19-13-18(14-22)21(25)23(2)15-19/h6-9,13,15-16H,3-5,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418927

(CHEMBL1807263)Show SMILES CCCNC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H22Cl2N2O2/c1-2-11-23-20(26)18(12-14-3-7-16(21)8-4-14)24-19(25)13-15-5-9-17(22)10-6-15/h3-10,18H,2,11-13H2,1H3,(H,23,26)(H,24,25)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 4622-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.092
BindingDB Entry DOI: 10.7270/Q20866KR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352798

(CHEMBL1823402)Show InChI InChI=1S/C20H25N3O2/c1-22-20(24)10-9-19(21-22)15-5-7-17(8-6-15)25-18-11-13-23(14-12-18)16-3-2-4-16/h5-10,16,18H,2-4,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from rat histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 1504-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.026
BindingDB Entry DOI: 10.7270/Q2W37WSF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50079576
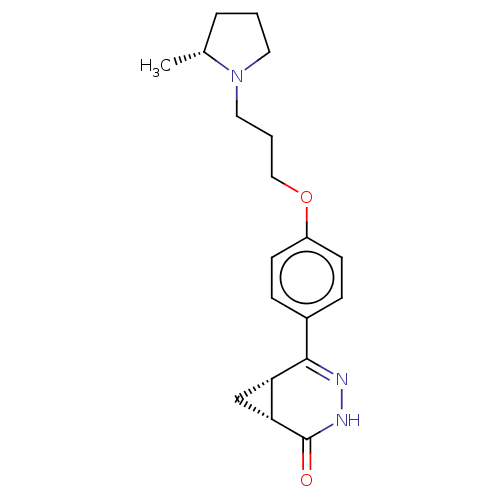
(CHEMBL3417585)Show SMILES [H][C@@]12C[C@]1([H])C(=NNC2=O)c1ccc(OCCCN2CCC[C@H]2C)cc1 |r,c:6| Show InChI InChI=1S/C19H25N3O2/c1-13-4-2-9-22(13)10-3-11-24-15-7-5-14(6-8-15)18-16-12-17(16)19(23)21-20-18/h5-8,13,16-17H,2-4,9-12H2,1H3,(H,21,23)/t13-,16+,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Global R&D.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Eur J Med Chem 95: 349-56 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.054
BindingDB Entry DOI: 10.7270/Q2C24Z4M |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50358569
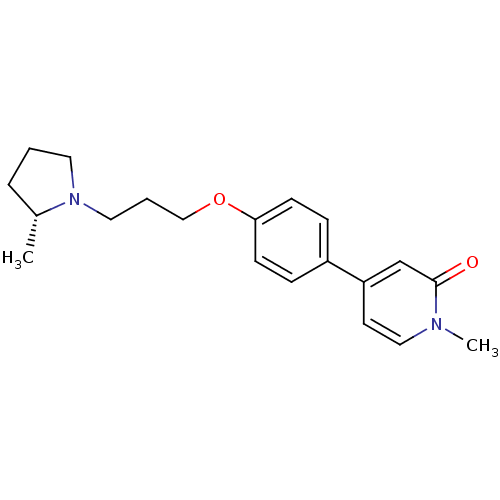
(CHEMBL1923730)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1ccn(C)c(=O)c1 |r| Show InChI InChI=1S/C20H26N2O2/c1-16-5-3-11-22(16)12-4-14-24-19-8-6-17(7-9-19)18-10-13-21(2)20(23)15-18/h6-10,13,15-16H,3-5,11-12,14H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50360889
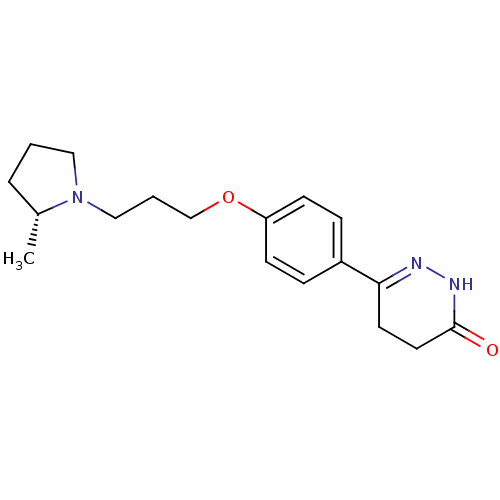
(CHEMBL1935100)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)CC1 |r,t:18| Show InChI InChI=1S/C18H25N3O2/c1-14-4-2-11-21(14)12-3-13-23-16-7-5-15(6-8-16)17-9-10-18(22)20-19-17/h5-8,14H,2-4,9-13H2,1H3,(H,20,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 22: 194-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.037
BindingDB Entry DOI: 10.7270/Q25Q4WJV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50358575

(CHEMBL1923736)Show SMILES C[C@@H]1CCCN1CCCOc1ccc(cc1)-c1cc(C#N)c(=O)n(C)c1 |r| Show InChI InChI=1S/C21H25N3O2/c1-16-5-3-10-24(16)11-4-12-26-20-8-6-17(7-9-20)19-13-18(14-22)21(25)23(2)15-19/h6-9,13,15-16H,3-5,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human histamine H3 receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 7076-80 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.091
BindingDB Entry DOI: 10.7270/Q26H4HVK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data