 Found 160 hits with Last Name = 'paulke' and Initial = 'a'
Found 160 hits with Last Name = 'paulke' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50426502
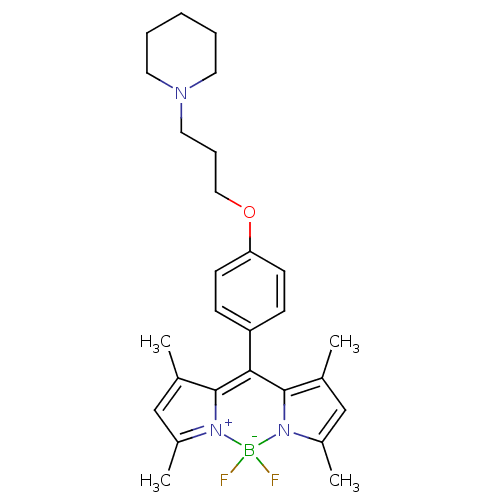
(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in HEK-293 cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394646

(CHEMBL2164608)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C30H26N2O4/c1-35-29-19-22(7-6-11-23-8-2-3-9-24(23)14-16-30(33)34)13-15-28(29)36-18-17-32-21-25-10-4-5-12-26(25)27(32)20-31/h2-6,8-16,19,21H,7,17-18H2,1H3,(H,33,34)/b11-6+,16-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394647
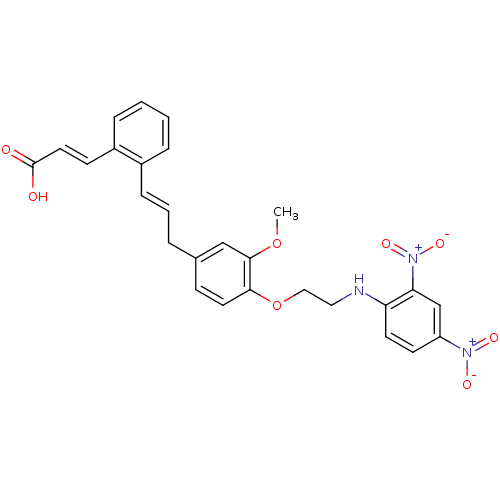
(CHEMBL2164610)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCCNc1ccc(cc1[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C27H25N3O8/c1-37-26-17-19(5-4-8-20-6-2-3-7-21(20)10-14-27(31)32)9-13-25(26)38-16-15-28-23-12-11-22(29(33)34)18-24(23)30(35)36/h2-4,6-14,17-18,28H,5,15-16H2,1H3,(H,31,32)/b8-4+,14-10+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 23.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394645
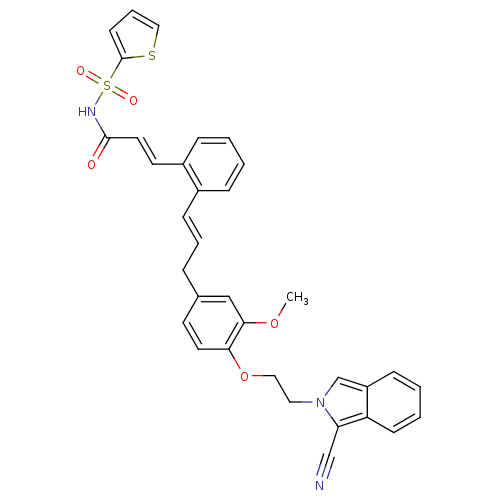
(CHEMBL2164609)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C34H29N3O5S2/c1-41-32-22-25(15-17-31(32)42-20-19-37-24-28-11-4-5-13-29(28)30(37)23-35)8-6-12-26-9-2-3-10-27(26)16-18-33(38)36-44(39,40)34-14-7-21-43-34/h2-7,9-18,21-22,24H,8,19-20H2,1H3,(H,36,38)/b12-6+,18-16+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from human recombinant histamine H3 receptor expressed in HEK-293 cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394644
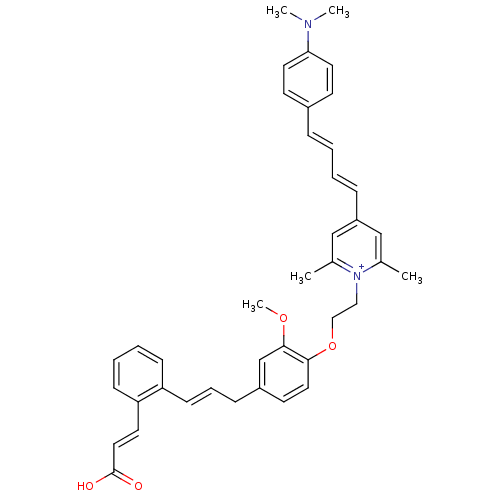
(CHEMBL2164612)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCC[n+]1c(C)cc(\C=C\C=C\c2ccc(cc2)N(C)C)cc1C Show InChI InChI=1S/C40H42N2O4/c1-30-27-34(12-7-6-11-32-17-21-37(22-18-32)41(3)4)28-31(2)42(30)25-26-46-38-23-19-33(29-39(38)45-5)13-10-16-35-14-8-9-15-36(35)20-24-40(43)44/h6-12,14-24,27-29H,13,25-26H2,1-5H3/p+1/b16-10+,24-20+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394648
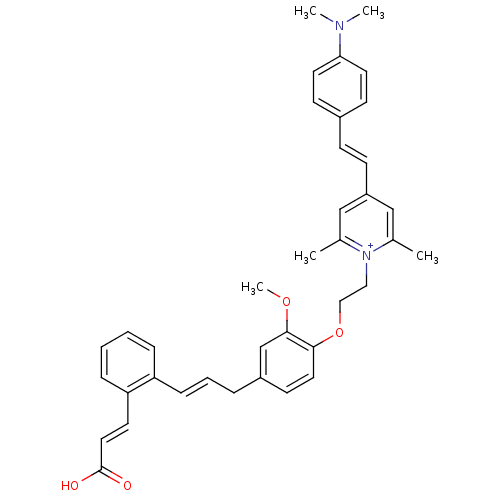
(CHEMBL2164611)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCC[n+]1c(C)cc(\C=C\c2ccc(cc2)N(C)C)cc1C Show InChI InChI=1S/C38H40N2O4/c1-28-25-32(14-13-30-15-19-35(20-16-30)39(3)4)26-29(2)40(28)23-24-44-36-21-17-31(27-37(36)43-5)9-8-12-33-10-6-7-11-34(33)18-22-38(41)42/h6-8,10-22,25-27H,9,23-24H2,1-5H3/p+1/b12-8+,22-18+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394649
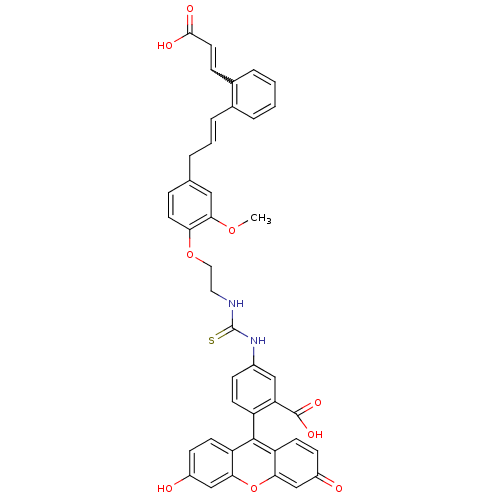
(CHEMBL2164613)Show SMILES COc1cc(C\C=C\c2ccccc2C=CC(O)=O)ccc1OCCNC(=S)Nc1ccc(c(c1)C(O)=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12 |w:14.14,(66.15,-44.12,;64.82,-43.36,;64.81,-41.82,;66.15,-41.04,;66.14,-39.5,;67.47,-38.73,;68.81,-39.5,;68.81,-41.04,;70.14,-41.81,;70.14,-43.35,;71.48,-44.12,;72.81,-43.35,;72.81,-41.81,;71.46,-41.04,;71.46,-39.5,;72.8,-38.73,;72.8,-37.19,;74.13,-36.42,;71.46,-36.42,;64.8,-38.74,;63.47,-39.51,;63.48,-41.05,;62.15,-41.82,;60.81,-41.06,;59.48,-41.83,;58.14,-41.06,;56.81,-41.83,;56.82,-43.37,;55.48,-41.07,;54.14,-41.84,;52.81,-41.07,;51.48,-41.84,;51.48,-43.38,;52.82,-44.15,;54.15,-43.38,;53.08,-45.67,;54.42,-46.43,;51.91,-46.66,;49.35,-44.62,;48.01,-43.85,;48.01,-42.31,;46.67,-41.55,;45.34,-42.32,;44.01,-41.55,;45.35,-43.86,;46.68,-44.63,;46.69,-46.17,;48.02,-46.94,;48.02,-48.48,;49.36,-49.24,;49.36,-50.78,;50.69,-48.47,;50.68,-46.93,;49.35,-46.16,)| Show InChI InChI=1S/C42H34N2O9S/c1-51-38-21-25(5-4-8-26-6-2-3-7-27(26)10-18-39(47)48)9-17-35(38)52-20-19-43-42(54)44-28-11-14-31(34(22-28)41(49)50)40-32-15-12-29(45)23-36(32)53-37-24-30(46)13-16-33(37)40/h2-4,6-18,21-24,45H,5,19-20H2,1H3,(H,47,48)(H,49,50)(H2,43,44,54)/b8-4+,18-10? | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 678 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50394644

(CHEMBL2164612)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCC[n+]1c(C)cc(\C=C\C=C\c2ccc(cc2)N(C)C)cc1C Show InChI InChI=1S/C40H42N2O4/c1-30-27-34(12-7-6-11-32-17-21-37(22-18-32)41(3)4)28-31(2)42(30)25-26-46-38-23-19-33(29-39(38)45-5)13-10-16-35-14-8-9-15-36(35)20-24-40(43)44/h6-12,14-24,27-29H,13,25-26H2,1-5H3/p+1/b16-10+,24-20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 902 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394650
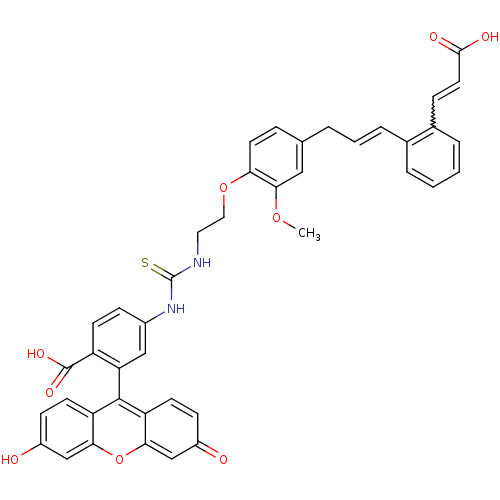
(CHEMBL2164614)Show SMILES COc1cc(C\C=C\c2ccccc2C=CC(O)=O)ccc1OCCNC(=S)Nc1ccc(C(O)=O)c(c1)-c1c2ccc(O)cc2oc2cc(=O)ccc12 |w:14.14,(31.57,-56.82,;30.24,-56.05,;30.24,-54.51,;31.57,-53.74,;31.57,-52.2,;32.91,-51.43,;34.24,-52.2,;35.58,-51.43,;36.91,-52.2,;36.91,-53.74,;38.24,-54.51,;39.58,-53.74,;39.58,-52.2,;38.24,-51.43,;38.24,-49.89,;39.58,-49.12,;39.58,-47.58,;40.91,-46.81,;38.24,-46.81,;30.24,-51.43,;28.91,-52.2,;28.91,-53.74,;27.57,-54.51,;26.24,-53.74,;24.91,-54.51,;23.58,-53.74,;22.25,-54.51,;22.25,-56.05,;20.9,-53.74,;19.58,-54.51,;19.58,-56.05,;18.24,-56.82,;16.91,-56.05,;15.58,-56.89,;15.58,-58.43,;14.25,-56.12,;16.91,-54.51,;18.24,-53.74,;14.78,-53.28,;14.78,-51.74,;16.11,-50.97,;16.11,-49.43,;14.78,-48.66,;14.78,-47.12,;13.44,-49.43,;13.44,-50.97,;12.11,-51.74,;12.11,-53.28,;10.77,-54.05,;10.77,-55.59,;9.44,-56.36,;12.11,-56.36,;13.44,-55.59,;13.44,-54.05,)| Show InChI InChI=1S/C42H34N2O9S/c1-51-38-21-25(5-4-8-26-6-2-3-7-27(26)10-18-39(47)48)9-17-35(38)52-20-19-43-42(54)44-28-11-14-31(41(49)50)34(22-28)40-32-15-12-29(45)23-36(32)53-37-24-30(46)13-16-33(37)40/h2-4,6-18,21-24,45H,5,19-20H2,1H3,(H,47,48)(H,49,50)(H2,43,44,54)/b8-4+,18-10? | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50426502

(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D3 receptor expressed in CHO-K1 cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50394651
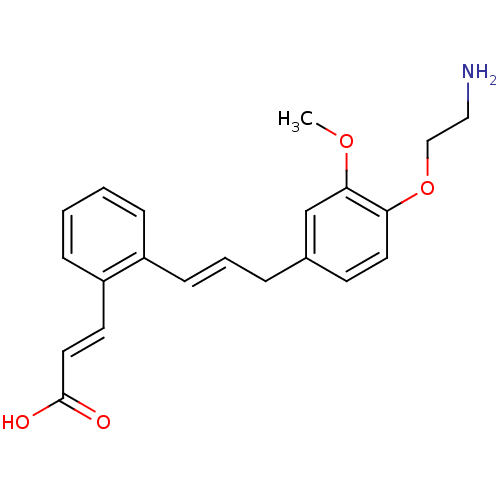
(CHEMBL2164607)Show InChI InChI=1S/C21H23NO4/c1-25-20-15-16(9-11-19(20)26-14-13-22)5-4-8-17-6-2-3-7-18(17)10-12-21(23)24/h2-4,6-12,15H,5,13-14,22H2,1H3,(H,23,24)/b8-4+,12-10+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50426502

(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO-K1 cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50159774
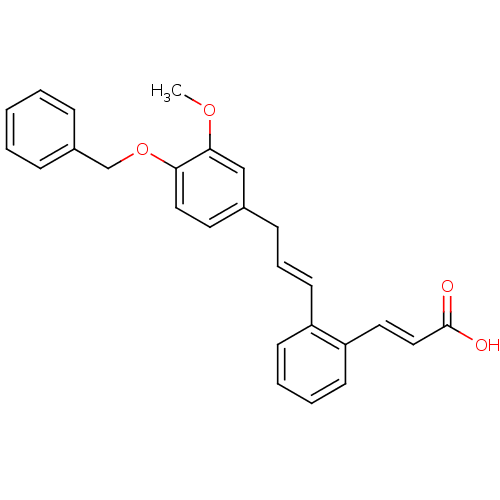
((E)-3-{2-[(E)-3-(4-Benzyloxy-3-methoxy-phenyl)-pro...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-9,11-18H,10,19H2,1H3,(H,27,28)/b13-7+,17-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50426502

(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from human dopamine D2short receptor expressed in CHO-K1 cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50193918
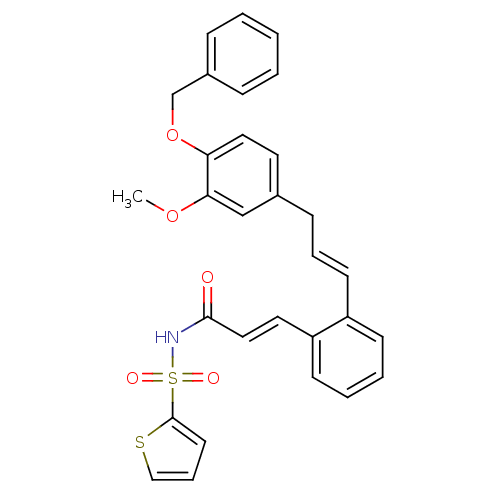
(3-(2-((E)-3-(4-(benzyloxy)-3-methoxyphenyl)prop-1-...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-10,12-21H,11,22H2,1H3,(H,31,32)/b14-7+,19-17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50193918

(3-(2-((E)-3-(4-(benzyloxy)-3-methoxyphenyl)prop-1-...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-10,12-21H,11,22H2,1H3,(H,31,32)/b14-7+,19-17+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50394644

(CHEMBL2164612)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCC[n+]1c(C)cc(\C=C\C=C\c2ccc(cc2)N(C)C)cc1C Show InChI InChI=1S/C40H42N2O4/c1-30-27-34(12-7-6-11-32-17-21-37(22-18-32)41(3)4)28-31(2)42(30)25-26-46-38-23-19-33(29-39(38)45-5)13-10-16-35-14-8-9-15-36(35)20-24-40(43)44/h6-12,14-24,27-29H,13,25-26H2,1-5H3/p+1/b16-10+,24-20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50394646

(CHEMBL2164608)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C30H26N2O4/c1-35-29-19-22(7-6-11-23-8-2-3-9-24(23)14-16-30(33)34)13-15-28(29)36-18-17-32-21-25-10-4-5-12-26(25)27(32)20-31/h2-6,8-16,19,21H,7,17-18H2,1H3,(H,33,34)/b11-6+,16-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50394646

(CHEMBL2164608)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C30H26N2O4/c1-35-29-19-22(7-6-11-23-8-2-3-9-24(23)14-16-30(33)34)13-15-28(29)36-18-17-32-21-25-10-4-5-12-26(25)27(32)20-31/h2-6,8-16,19,21H,7,17-18H2,1H3,(H,33,34)/b11-6+,16-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50193918

(3-(2-((E)-3-(4-(benzyloxy)-3-methoxyphenyl)prop-1-...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCc1ccccc1 Show InChI InChI=1S/C30H27NO5S2/c1-35-28-21-23(16-18-27(28)36-22-24-9-3-2-4-10-24)11-7-14-25-12-5-6-13-26(25)17-19-29(32)31-38(33,34)30-15-8-20-37-30/h2-10,12-21H,11,22H2,1H3,(H,31,32)/b14-7+,19-17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50426502

(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human dopamine D5 receptor expressed in HEK cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50159774

((E)-3-{2-[(E)-3-(4-Benzyloxy-3-methoxy-phenyl)-pro...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-9,11-18H,10,19H2,1H3,(H,27,28)/b13-7+,17-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50426502

(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in Sf9 cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50394645

(CHEMBL2164609)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C34H29N3O5S2/c1-41-32-22-25(15-17-31(32)42-20-19-37-24-28-11-4-5-13-29(28)30(37)23-35)8-6-12-26-9-2-3-10-27(26)16-18-33(38)36-44(39,40)34-14-7-21-43-34/h2-7,9-18,21-22,24H,8,19-20H2,1H3,(H,36,38)/b12-6+,18-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50394645

(CHEMBL2164609)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C34H29N3O5S2/c1-41-32-22-25(15-17-31(32)42-20-19-37-24-28-11-4-5-13-29(28)30(37)23-35)8-6-12-26-9-2-3-10-27(26)16-18-33(38)36-44(39,40)34-14-7-21-43-34/h2-7,9-18,21-22,24H,8,19-20H2,1H3,(H,36,38)/b12-6+,18-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50426502

(Bodilisant | CHEMBL2323582)Show SMILES CC1=CC(C)=[N+]2C1=C(c1c(C)cc(C)n1[B-]2(F)F)c1ccc(OCCCN2CCCCC2)cc1 |c:4,t:1,7| Show InChI InChI=1S/C27H34BF2N3O/c1-19-17-21(3)32-26(19)25(27-20(2)18-22(4)33(27)28(32,29)30)23-9-11-24(12-10-23)34-16-8-15-31-13-6-5-7-14-31/h9-12,17-18H,5-8,13-16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK cells |
ACS Med Chem Lett 4: 269-73 (2013)
Article DOI: 10.1021/ml300383n
BindingDB Entry DOI: 10.7270/Q2N87C3T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50394646

(CHEMBL2164608)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C30H26N2O4/c1-35-29-19-22(7-6-11-23-8-2-3-9-24(23)14-16-30(33)34)13-15-28(29)36-18-17-32-21-25-10-4-5-12-26(25)27(32)20-31/h2-6,8-16,19,21H,7,17-18H2,1H3,(H,33,34)/b11-6+,16-14+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50394645

(CHEMBL2164609)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(=O)NS(=O)(=O)c2cccs2)ccc1OCCn1cc2ccccc2c1C#N Show InChI InChI=1S/C34H29N3O5S2/c1-41-32-22-25(15-17-31(32)42-20-19-37-24-28-11-4-5-13-29(28)30(37)23-35)8-6-12-26-9-2-3-10-27(26)16-18-33(38)36-44(39,40)34-14-7-21-43-34/h2-7,9-18,21-22,24H,8,19-20H2,1H3,(H,36,38)/b12-6+,18-16+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50394644

(CHEMBL2164612)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCC[n+]1c(C)cc(\C=C\C=C\c2ccc(cc2)N(C)C)cc1C Show InChI InChI=1S/C40H42N2O4/c1-30-27-34(12-7-6-11-32-17-21-37(22-18-32)41(3)4)28-31(2)42(30)25-26-46-38-23-19-33(29-39(38)45-5)13-10-16-35-14-8-9-15-36(35)20-24-40(43)44/h6-12,14-24,27-29H,13,25-26H2,1-5H3/p+1/b16-10+,24-20+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50159774

((E)-3-{2-[(E)-3-(4-Benzyloxy-3-methoxy-phenyl)-pro...)Show SMILES COc1cc(C\C=C\c2ccccc2\C=C\C(O)=O)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H24O4/c1-29-25-18-20(14-16-24(25)30-19-21-8-3-2-4-9-21)10-7-13-22-11-5-6-12-23(22)15-17-26(27)28/h2-9,11-18H,10,19H2,1H3,(H,27,28)/b13-7+,17-15+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1R expressed in chem1 cells after 2hrs by beta counting |
ACS Med Chem Lett 3: 774-779 (2012)
Article DOI: 10.1021/ml300191g
BindingDB Entry DOI: 10.7270/Q2DZ09D3 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241116

(CHEMBL4066332)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NS(C)(=O)=O)cc1Cl Show InChI InChI=1S/C19H23ClN2O3S/c1-19(2,3)15-8-5-13(6-9-15)18(23)21-12-14-7-10-16(11-17(14)20)22-26(4,24)25/h5-11,22H,12H2,1-4H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of sEH in human HepG2 cells using 14(15)-EET-d11 as substrate assessed as reduction in conversion of 14(15)-EET-d11 to 14(15)-DHET-d11 pre... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241116

(CHEMBL4066332)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NS(C)(=O)=O)cc1Cl Show InChI InChI=1S/C19H23ClN2O3S/c1-19(2,3)15-8-5-13(6-9-15)18(23)21-12-14-7-10-16(11-17(14)20)22-26(4,24)25/h5-11,22H,12H2,1-4H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant v-Abl tyrosine kinase. |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241151
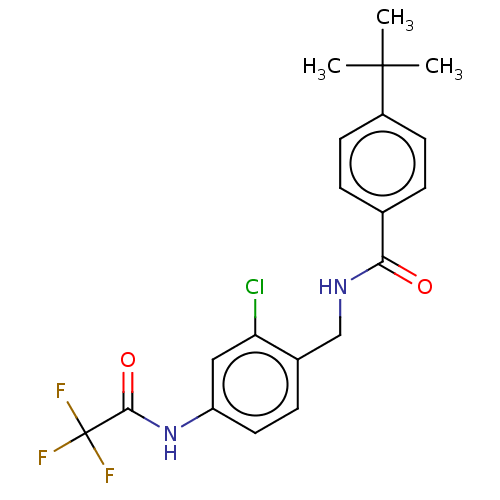
(CHEMBL4087681)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NC(=O)C(F)(F)F)cc1Cl Show InChI InChI=1S/C20H20ClF3N2O2/c1-19(2,3)14-7-4-12(5-8-14)17(27)25-11-13-6-9-15(10-16(13)21)26-18(28)20(22,23)24/h4-10H,11H2,1-3H3,(H,25,27)(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241160

(CHEMBL4095371)Show InChI InChI=1S/C18H21ClN2O/c1-18(2,3)14-7-4-12(5-8-14)17(22)21-11-13-6-9-15(20)10-16(13)19/h4-10H,11,20H2,1-3H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241158
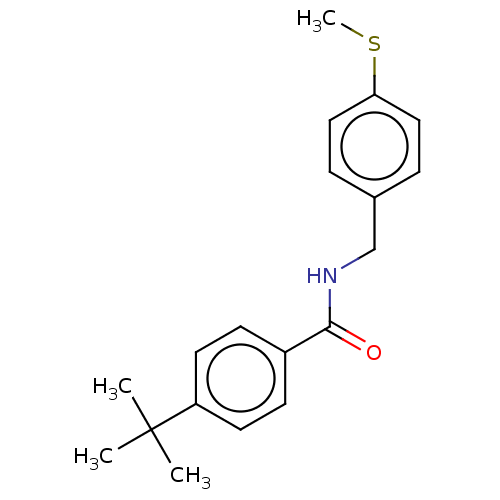
(CHEMBL4064739)Show InChI InChI=1S/C19H23NOS/c1-19(2,3)16-9-7-15(8-10-16)18(21)20-13-14-5-11-17(22-4)12-6-14/h5-12H,13H2,1-4H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241150
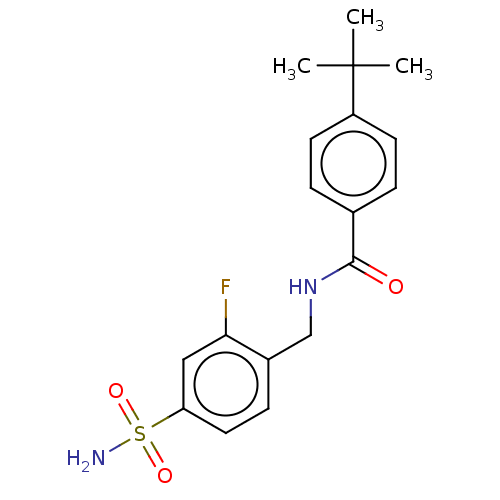
(CHEMBL4094881)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(cc1F)S(N)(=O)=O Show InChI InChI=1S/C18H21FN2O3S/c1-18(2,3)14-7-4-12(5-8-14)17(22)21-11-13-6-9-15(10-16(13)19)25(20,23)24/h4-10H,11H2,1-3H3,(H,21,22)(H2,20,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241109

(CHEMBL4076701)Show InChI InChI=1S/C20H23NO2/c1-14(22)16-7-5-15(6-8-16)13-21-19(23)17-9-11-18(12-10-17)20(2,3)4/h5-12H,13H2,1-4H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241136
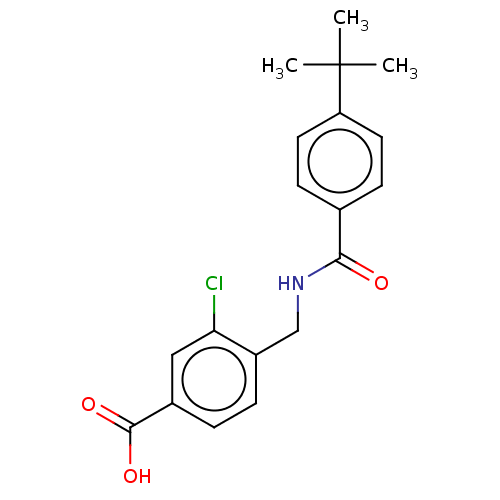
(CHEMBL4088582)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C19H20ClNO3/c1-19(2,3)15-8-6-12(7-9-15)17(22)21-11-14-5-4-13(18(23)24)10-16(14)20/h4-10H,11H2,1-3H3,(H,21,22)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241146

(CHEMBL4092436)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C19H20F3NO2/c1-18(2,3)15-8-6-14(7-9-15)17(24)23-12-13-4-10-16(11-5-13)25-19(20,21)22/h4-11H,12H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241147

(CHEMBL4084686)Show SMILES C[S+]([O-])c1ccc(CNC(=O)c2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C19H23NO2S/c1-19(2,3)16-9-7-15(8-10-16)18(21)20-13-14-5-11-17(12-6-14)23(4)22/h5-12H,13H2,1-4H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM10894

(4-(4-tert-Butylphenylcarboxamidomethyl)benzenesulf...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H22N2O3S/c1-18(2,3)15-8-6-14(7-9-15)17(21)20-12-13-4-10-16(11-5-13)24(19,22)23/h4-11H,12H2,1-3H3,(H,20,21)(H2,19,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241176
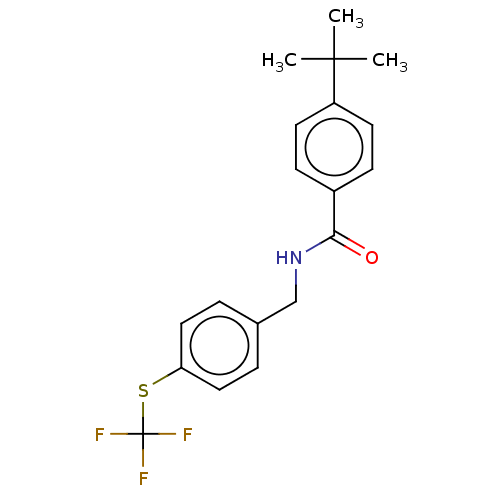
(CHEMBL4074393)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(SC(F)(F)F)cc1 Show InChI InChI=1S/C19H20F3NOS/c1-18(2,3)15-8-6-14(7-9-15)17(24)23-12-13-4-10-16(11-5-13)25-19(20,21)22/h4-11H,12H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241144
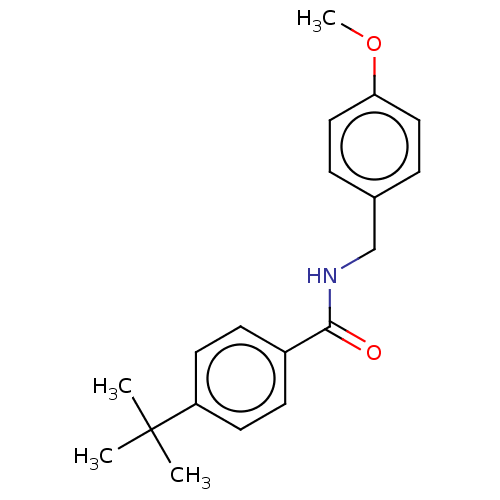
(CHEMBL1300445)Show InChI InChI=1S/C19H23NO2/c1-19(2,3)16-9-7-15(8-10-16)18(21)20-13-14-5-11-17(22-4)12-6-14/h5-12H,13H2,1-4H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant v-Abl tyrosine kinase. |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241104
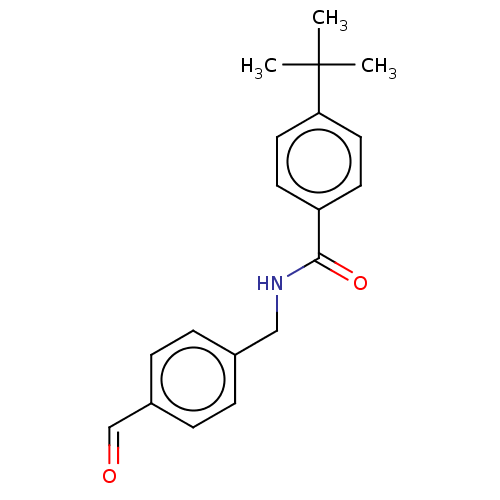
(CHEMBL4091019)Show InChI InChI=1S/C19H21NO2/c1-19(2,3)17-10-8-16(9-11-17)18(22)20-12-14-4-6-15(13-21)7-5-14/h4-11,13H,12H2,1-3H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241145
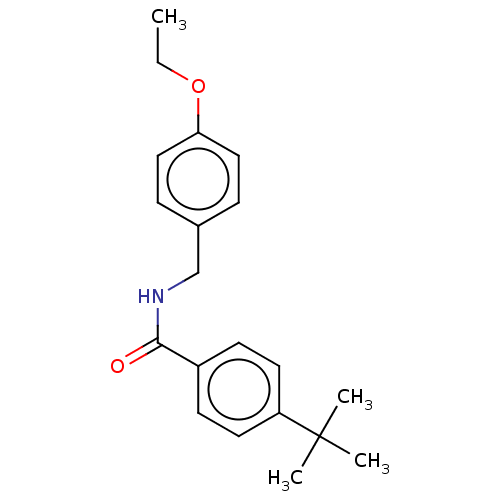
(CHEMBL4100178)Show InChI InChI=1S/C20H25NO2/c1-5-23-18-12-6-15(7-13-18)14-21-19(22)16-8-10-17(11-9-16)20(2,3)4/h6-13H,5,14H2,1-4H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant v-Abl tyrosine kinase. |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
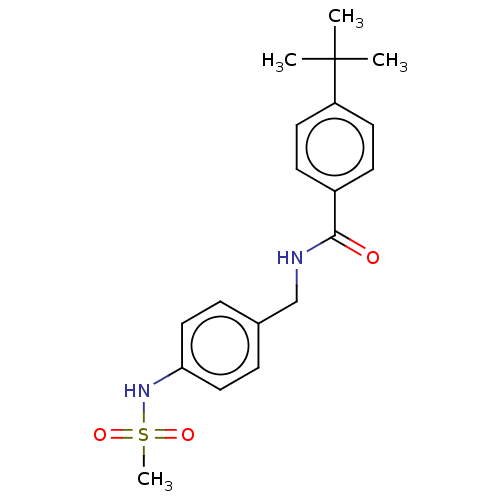
(Homo sapiens (Human)) | BDBM50241168
(CHEMBL4061980)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H24N2O3S/c1-19(2,3)16-9-7-15(8-10-16)18(22)20-13-14-5-11-17(12-6-14)21-25(4,23)24/h5-12,21H,13H2,1-4H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
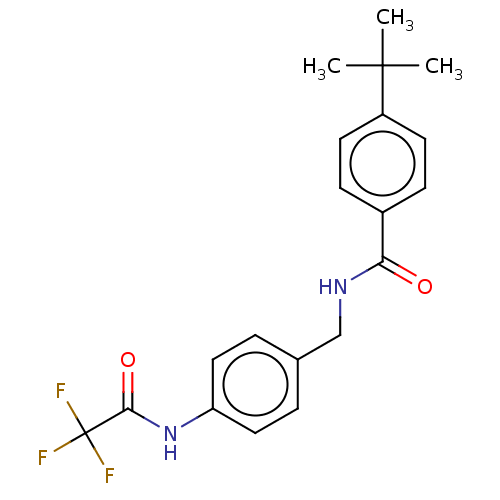
(Homo sapiens (Human)) | BDBM50241086
(CHEMBL4071919)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(NC(=O)C(F)(F)F)cc1 Show InChI InChI=1S/C20H21F3N2O2/c1-19(2,3)15-8-6-14(7-9-15)17(26)24-12-13-4-10-16(11-5-13)25-18(27)20(21,22)23/h4-11H,12H2,1-3H3,(H,24,26)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241139
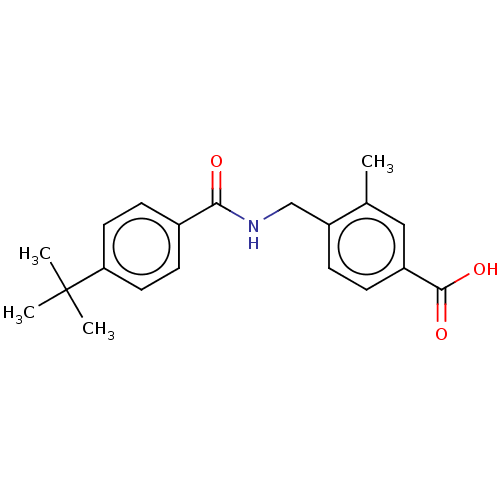
(CHEMBL4076274)Show InChI InChI=1S/C20H23NO3/c1-13-11-15(19(23)24)5-6-16(13)12-21-18(22)14-7-9-17(10-8-14)20(2,3)4/h5-11H,12H2,1-4H3,(H,21,22)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50241141

(CHEMBL4083383)Show InChI InChI=1S/C19H20N2O/c1-19(2,3)17-10-8-16(9-11-17)18(22)21-13-15-6-4-14(12-20)5-7-15/h4-11H,13H2,1-3H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sEH using PHOME as substrate preincubated for 30 mins followed by substrate addition measured for 30 mins by fluoresc... |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data