 Found 6251 hits with Last Name = 'powell' and Initial = 'a'
Found 6251 hits with Last Name = 'powell' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50463046
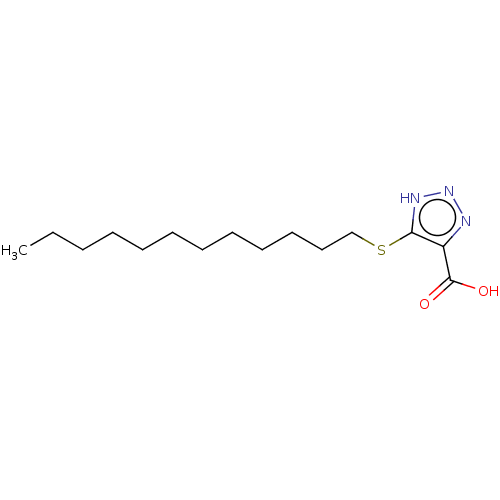
(CHEMBL1229989)Show InChI InChI=1S/C15H27N3O2S/c1-2-3-4-5-6-7-8-9-10-11-12-21-14-13(15(19)20)16-18-17-14/h2-12H2,1H3,(H,19,20)(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human glycolate oxidase expressed in Escherichia coli using glycolate as substrate by DCIP dye based spectrophotometry analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463047

(CHEMBL1794748)Show InChI InChI=1S/C9H5ClN2O2S2/c10-5-1-3-6(4-2-5)15-9-7(8(13)14)11-12-16-9/h1-4H,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non competitive inhibition of mouse glycolate oxidase in hyperoxaluric-Agxt knockdown mouse primary hepatocyte assessed as reduction in oxalate produ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18012
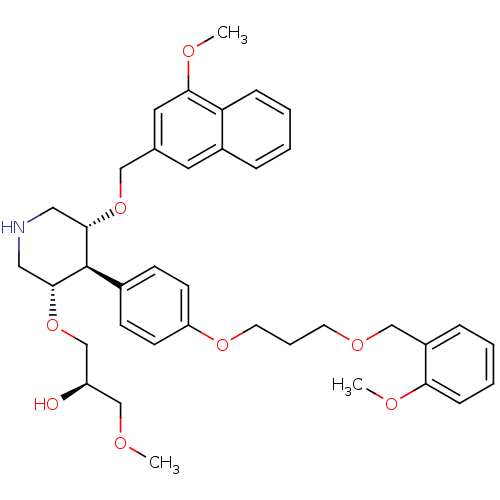
(trans,trans-4-arylpiperidine-based compound, 1)Show SMILES COC[C@@H](O)CO[C@@H]1CNC[C@H](OCc2cc(OC)c3ccccc3c2)[C@H]1c1ccc(OCCCOCc2ccccc2OC)cc1 |r| Show InChI InChI=1S/C38H47NO8/c1-41-25-31(40)26-47-37-22-39-21-36(46-23-27-19-29-9-4-6-11-33(29)35(20-27)43-3)38(37)28-13-15-32(16-14-28)45-18-8-17-44-24-30-10-5-7-12-34(30)42-2/h4-7,9-16,19-20,31,36-40H,8,17-18,21-26H2,1-3H3/t31-,36+,37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 17: 3575-80 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.052
BindingDB Entry DOI: 10.7270/Q2B56H0X |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50572932

(CHEMBL4850573)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(Oc3nn[nH]c3C(O)=O)cc2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50250656

(CHEMBL4081890 | US11247971, Cmpd ID 276)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(c2)-c2ccccc2)-c2nc(cs2)C(O)=O)cc1 Show InChI InChI=1S/C30H26N4O4S2/c31-40(37,38)24-13-11-19(12-14-24)15-25-27(16-20-9-10-20)34(30-32-26(18-39-30)29(35)36)33-28(25)23-8-4-7-22(17-23)21-5-2-1-3-6-21/h1-8,11-14,17-18,20H,9-10,15-16H2,(H,35,36)(H2,31,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50572931
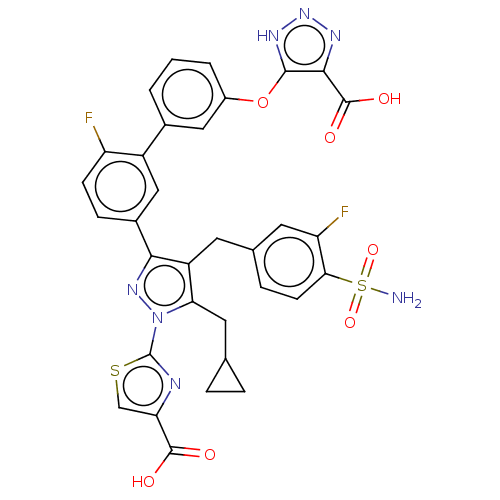
(CHEMBL4855986)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2cccc(Oc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18033
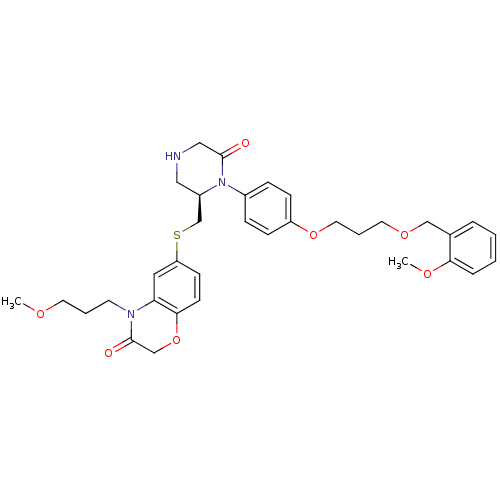
(Ketopiperazine-based inhibitor, 13)Show SMILES COCCCN1C(=O)COc2ccc(SC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C34H41N3O7S/c1-40-16-5-15-36-30-19-29(13-14-32(30)44-23-34(36)39)45-24-27-20-35-21-33(38)37(27)26-9-11-28(12-10-26)43-18-6-17-42-22-25-7-3-4-8-31(25)41-2/h3-4,7-14,19,27,35H,5-6,15-18,20-24H2,1-2H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50572931

(CHEMBL4855986)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2cccc(Oc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50572933

(CHEMBL4861379)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(Sc3nn[nH]c3C(O)=O)cc2)-c2nc(cs2)C(O)=O)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50572930

(CHEMBL4873879)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(Oc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50572934

(CHEMBL4847068)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(cc2)-c2nn[nH]c2C(O)=O)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17967
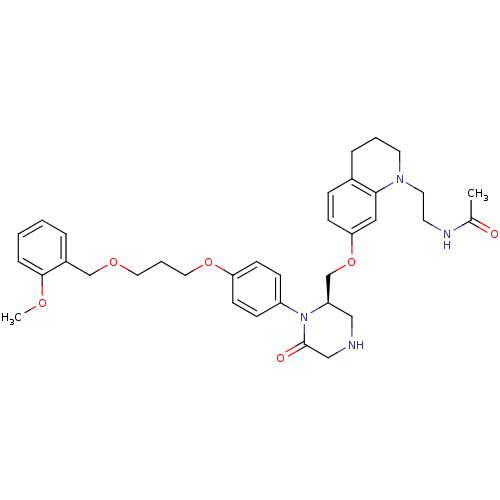
(CHEMBL411885 | Ketopiperazine-based compound, 16 |...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCNC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H44N4O6/c1-26(40)37-16-18-38-17-5-8-27-10-13-32(21-33(27)38)45-25-30-22-36-23-35(41)39(30)29-11-14-31(15-12-29)44-20-6-19-43-24-28-7-3-4-9-34(28)42-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3,(H,37,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50572933

(CHEMBL4861379)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(Sc3nn[nH]c3C(O)=O)cc2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50572934

(CHEMBL4847068)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(cc2)-c2nn[nH]c2C(O)=O)-c2nc(cs2)C(O)=O)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50572932

(CHEMBL4850573)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(Oc3nn[nH]c3C(O)=O)cc2)-c2nc(cs2)C(O)=O)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50572930

(CHEMBL4873879)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(Oc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17967

(CHEMBL411885 | Ketopiperazine-based compound, 16 |...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCNC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H44N4O6/c1-26(40)37-16-18-38-17-5-8-27-10-13-32(21-33(27)38)45-25-30-22-36-23-35(41)39(30)29-11-14-31(15-12-29)44-20-6-19-43-24-28-7-3-4-9-34(28)42-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3,(H,37,40)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against renin in fluorescent tGFP assay |
Bioorg Med Chem Lett 15: 4713-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.063
BindingDB Entry DOI: 10.7270/Q2SF2VP9 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18025
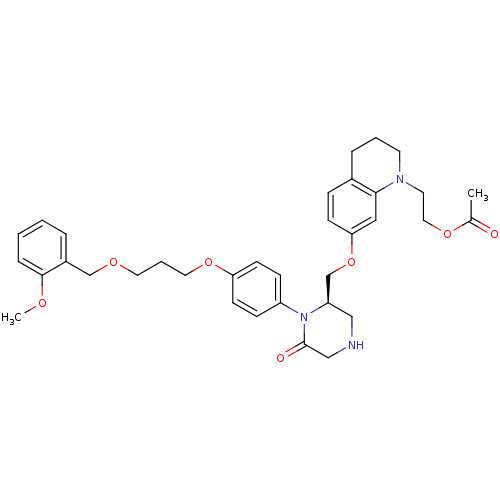
(2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]prop...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3CCCN(CCOC(C)=O)c3c2)CNCC1=O |r| Show InChI InChI=1S/C35H43N3O7/c1-26(39)43-20-17-37-16-5-8-27-10-13-32(21-33(27)37)45-25-30-22-36-23-35(40)38(30)29-11-14-31(15-12-29)44-19-6-18-42-24-28-7-3-4-9-34(28)41-2/h3-4,7,9-15,21,30,36H,5-6,8,16-20,22-25H2,1-2H3/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50572929

(CHEMBL4864519)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(Sc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17965
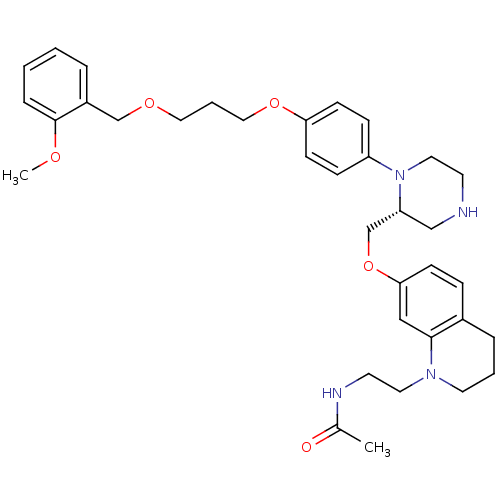
(N-[2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]p...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1CCNC[C@@H]1COc1ccc2CCCN(CCNC(C)=O)c2c1 |r| Show InChI InChI=1S/C35H46N4O5/c1-27(40)37-17-19-38-18-5-8-28-10-13-33(23-34(28)38)44-26-31-24-36-16-20-39(31)30-11-14-32(15-12-30)43-22-6-21-42-25-29-7-3-4-9-35(29)41-2/h3-4,7,9-15,23,31,36H,5-6,8,16-22,24-26H2,1-2H3,(H,37,40)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 13: 2657-64 (2005)
Article DOI: 10.1016/j.bmc.2005.01.048
BindingDB Entry DOI: 10.7270/Q2KP80D6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50258579
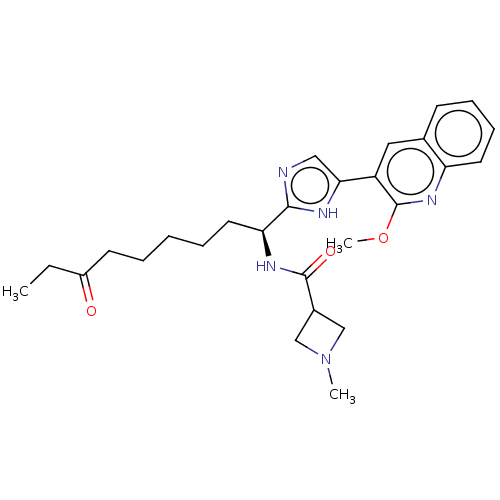
((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18032

(Ketopiperazine-based inhibitor, 12)Show SMILES COCCCN1C(=O)COc2ccc(OC[C@H]3CNCC(=O)N3c3ccc(OCCCOCc4ccccc4OC)cc3)cc12 |r| Show InChI InChI=1S/C34H41N3O8/c1-40-16-5-15-36-30-19-29(13-14-32(30)45-24-34(36)39)44-23-27-20-35-21-33(38)37(27)26-9-11-28(12-10-26)43-18-6-17-42-22-25-7-3-4-8-31(25)41-2/h3-4,7-14,19,27,35H,5-6,15-18,20-24H2,1-2H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem Lett 16: 2500-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.084
BindingDB Entry DOI: 10.7270/Q26D5R7R |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Mus musculus (Mouse)) | BDBM50572929

(CHEMBL4864519)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2cccc(Sc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425862
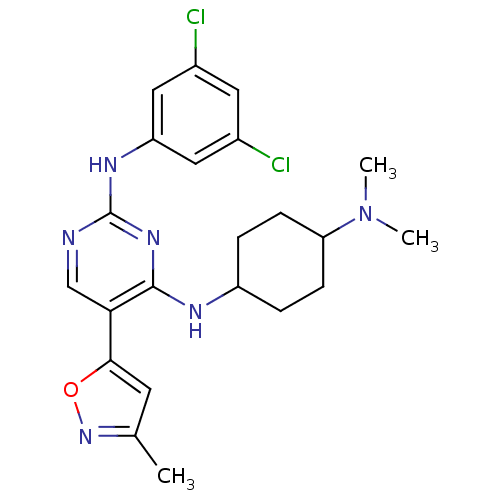
(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17996
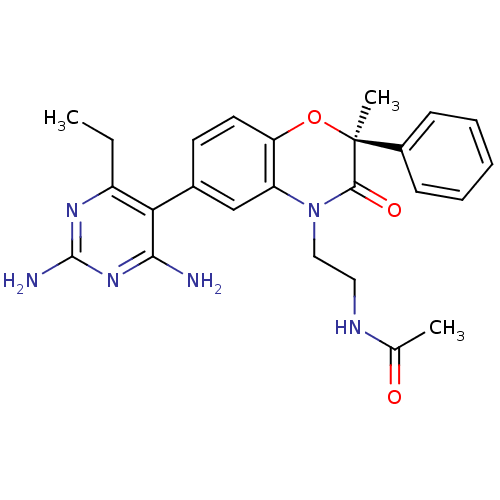
(1,4-benzoxazin-3-one, 33 | N-{2-[(2S)-6-(2,4-diami...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2O[C@](C)(C(=O)N(CCNC(C)=O)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C25H28N6O3/c1-4-18-21(22(26)30-24(27)29-18)16-10-11-20-19(14-16)31(13-12-28-15(2)32)23(33)25(3,34-20)17-8-6-5-7-9-17/h5-11,14H,4,12-13H2,1-3H3,(H,28,32)(H4,26,27,29,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 15: 5912-49 (2007)
Article DOI: 10.1016/j.bmc.2007.05.069
BindingDB Entry DOI: 10.7270/Q2FX77QX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50572931

(CHEMBL4855986)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2cccc(Oc3[nH]nnc3C(O)=O)c2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17989
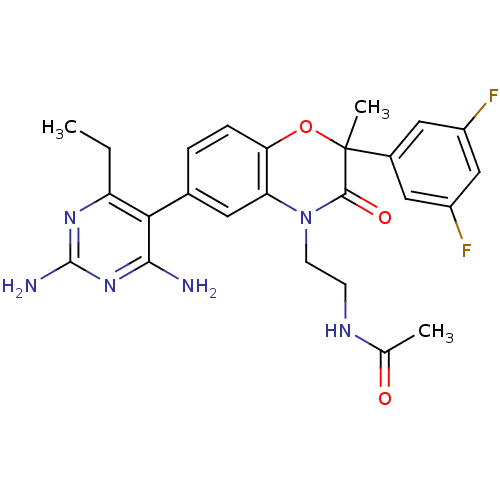
(1,4-benzoxazin-3-one, 26 | N-{2-[6-(2,4-diamino-6-...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2OC(C)(C(=O)N(CCNC(C)=O)c2c1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C25H26F2N6O3/c1-4-18-21(22(28)32-24(29)31-18)14-5-6-20-19(9-14)33(8-7-30-13(2)34)23(35)25(3,36-20)15-10-16(26)12-17(27)11-15/h5-6,9-12H,4,7-8H2,1-3H3,(H,30,34)(H4,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 15: 5912-49 (2007)
Article DOI: 10.1016/j.bmc.2007.05.069
BindingDB Entry DOI: 10.7270/Q2FX77QX |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50362592
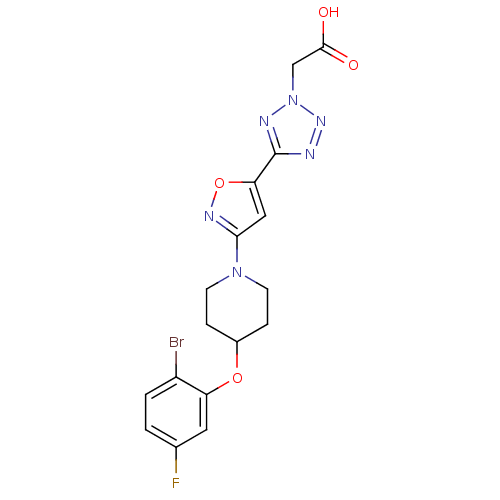
(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569630
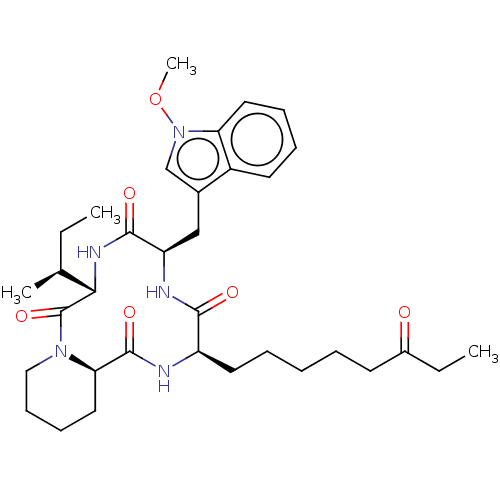
(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50572933

(CHEMBL4861379)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(Sc3nn[nH]c3C(O)=O)cc2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50572932

(CHEMBL4850573)Show SMILES NS(=O)(=O)c1ccc(Cc2c(CC3CC3)n(nc2-c2ccc(F)c(c2)-c2ccc(Oc3nn[nH]c3C(O)=O)cc2)-c2nc(cs2)C(O)=O)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after |
Bioorg Med Chem Lett 23: 767-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.088
BindingDB Entry DOI: 10.7270/Q2542PW7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569630

(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18005
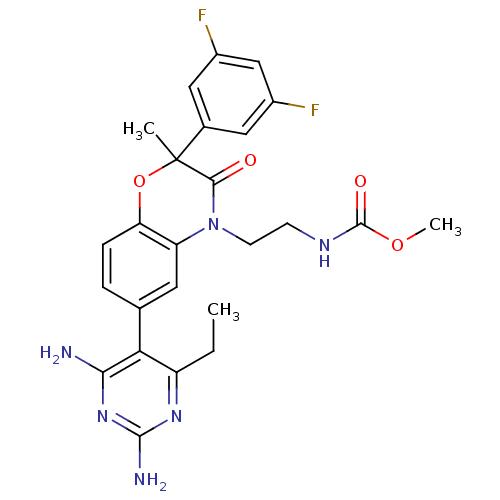
(1,4-benzoxazin-3-one, 42 | methyl N-{2-[6-(2,4-dia...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2OC(C)(C(=O)N(CCNC(=O)OC)c2c1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C25H26F2N6O4/c1-4-17-20(21(28)32-23(29)31-17)13-5-6-19-18(9-13)33(8-7-30-24(35)36-3)22(34)25(2,37-19)14-10-15(26)12-16(27)11-14/h5-6,9-12H,4,7-8H2,1-3H3,(H,30,35)(H4,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 15: 5912-49 (2007)
Article DOI: 10.1016/j.bmc.2007.05.069
BindingDB Entry DOI: 10.7270/Q2FX77QX |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50305768
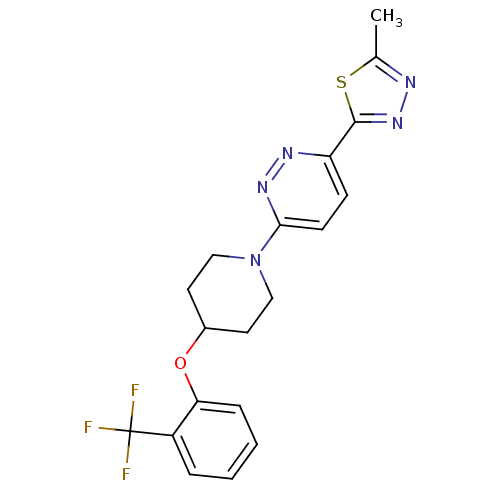
(2-methyl-5-(6-(4-(2-(trifluoromethyl)phenoxy)piper...)Show SMILES Cc1nnc(s1)-c1ccc(nn1)N1CCC(CC1)Oc1ccccc1C(F)(F)F Show InChI InChI=1S/C19H18F3N5OS/c1-12-23-26-18(29-12)15-6-7-17(25-24-15)27-10-8-13(9-11-27)28-16-5-3-2-4-14(16)19(20,21)22/h2-7,13H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425864
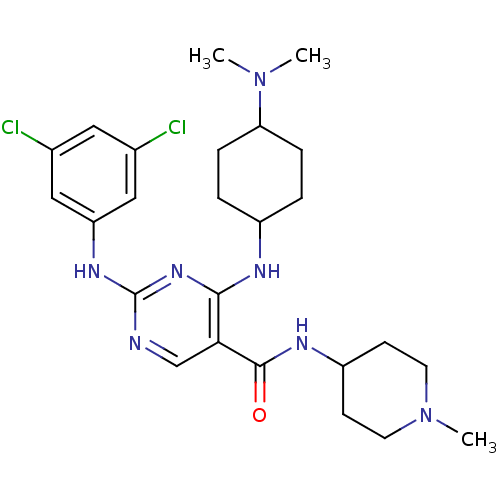
(CHEMBL2312649)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |(47.36,-51.36,;48.9,-51.37,;49.67,-52.7,;49.68,-50.04,;48.92,-48.7,;49.7,-47.37,;51.23,-47.39,;52.01,-48.71,;51.22,-50.05,;52,-46.06,;51.24,-44.72,;49.7,-44.72,;48.94,-43.38,;47.4,-43.38,;46.63,-42.04,;47.41,-40.71,;46.64,-39.37,;47.41,-38.04,;45.1,-39.37,;44.33,-40.71,;42.79,-40.71,;45.1,-42.04,;49.7,-42.05,;51.24,-42.05,;52.01,-43.39,;53.55,-43.39,;54.32,-44.72,;54.32,-42.06,;55.86,-42.06,;56.62,-43.39,;58.16,-43.4,;58.94,-42.07,;60.48,-42.08,;58.17,-40.73,;56.62,-40.72,)| Show InChI InChI=1S/C25H35Cl2N7O/c1-33(2)21-6-4-18(5-7-21)29-23-22(24(35)30-19-8-10-34(3)11-9-19)15-28-25(32-23)31-20-13-16(26)12-17(27)14-20/h12-15,18-19,21H,4-11H2,1-3H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50097721
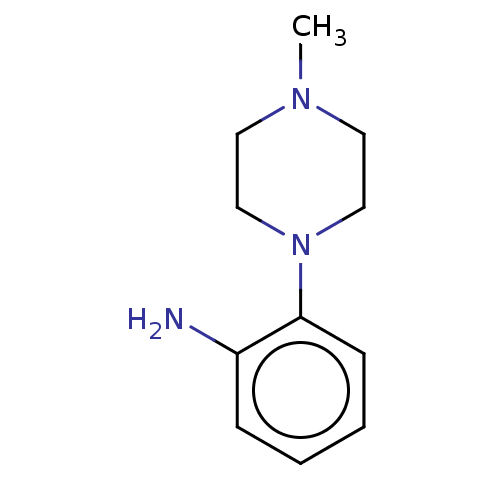
(CHEMBL1879790 | EN300-11843)Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-33(3)26(36)20-35-19-24(16-23-17-31-28(38)34(5-2)18-23)27(37)32-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258579

((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568
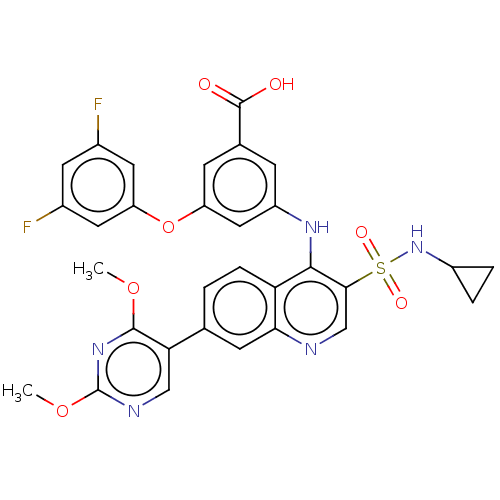
(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of recombinant human LDHA in the presence of NADH by resazurin dye reduction method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00196
BindingDB Entry DOI: 10.7270/Q2V128MV |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Serine protease HTRA1
(Homo sapiens (Human)) | BDBM560274
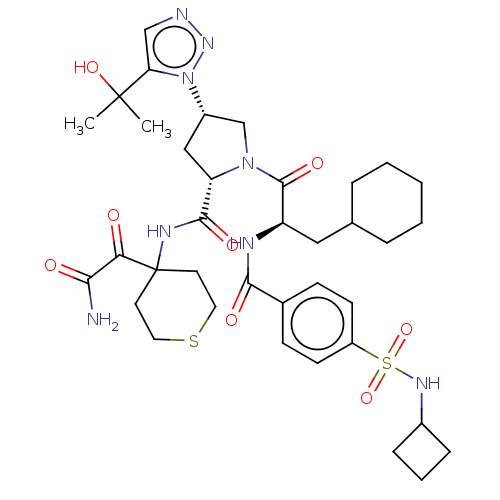
(US11377439, Example 111)Show SMILES CC(C)(O)c1cnnn1[C@H]1C[C@H](N(C1)C(=O)[C@@H](CC1CCCCC1)NC(=O)c1ccc(cc1)S(=O)(=O)NC1CCC1)C(=O)NC1(CCSCC1)C(=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z03CDC |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18001

(1,4-benzoxazin-3-one, 38 | N-{2-[6-(2,4-diamino-6-...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2OC(C)(C(=O)N(CCNC(=O)CO)c2c1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C25H26F2N6O4/c1-3-17-21(22(28)32-24(29)31-17)13-4-5-19-18(8-13)33(7-6-30-20(35)12-34)23(36)25(2,37-19)14-9-15(26)11-16(27)10-14/h4-5,8-11,34H,3,6-7,12H2,1-2H3,(H,30,35)(H4,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... |
Bioorg Med Chem 15: 5912-49 (2007)
Article DOI: 10.1016/j.bmc.2007.05.069
BindingDB Entry DOI: 10.7270/Q2FX77QX |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of rat SCD-1 |
J Med Chem 54: 5082-96 (2011)
Article DOI: 10.1021/jm200319u
BindingDB Entry DOI: 10.7270/Q2348MGP |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50362592

(CHEMBL1938870)Show SMILES OC(=O)Cn1nnc(n1)-c1cc(no1)N1CCC(CC1)Oc1cc(F)ccc1Br Show InChI InChI=1S/C17H16BrFN6O4/c18-12-2-1-10(19)7-13(12)28-11-3-5-24(6-4-11)15-8-14(29-22-15)17-20-23-25(21-17)9-16(26)27/h1-2,7-8,11H,3-6,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse SCD-1 |
Bioorg Med Chem Lett 22: 980-4 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.002
BindingDB Entry DOI: 10.7270/Q2833SHH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425870
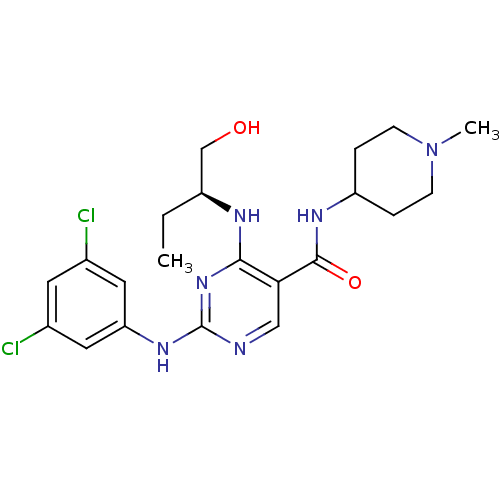
(CHEMBL2311550)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C21H28Cl2N6O2/c1-3-15(12-30)25-19-18(20(31)26-16-4-6-29(2)7-5-16)11-24-21(28-19)27-17-9-13(22)8-14(23)10-17/h8-11,15-16,30H,3-7,12H2,1-2H3,(H,26,31)(H2,24,25,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data