 Found 1775 hits with Last Name = 'strack' and Initial = 'a'
Found 1775 hits with Last Name = 'strack' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245192
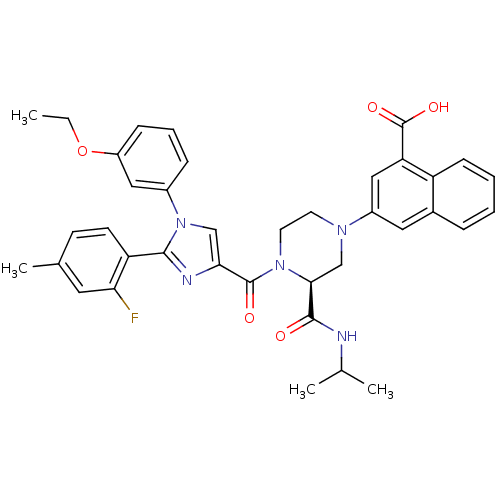
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H38FN5O5/c1-5-49-28-11-8-10-26(19-28)44-21-33(41-35(44)30-14-13-24(4)17-32(30)39)37(46)43-16-15-42(22-34(43)36(45)40-23(2)3)27-18-25-9-6-7-12-29(25)31(20-27)38(47)48/h6-14,17-21,23,34H,5,15-16,22H2,1-4H3,(H,40,45)(H,47,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245186
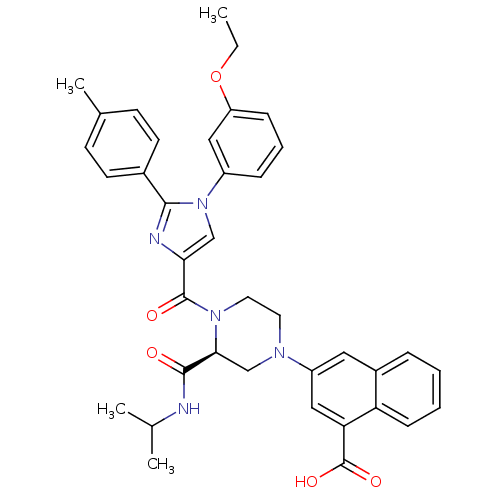
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245205
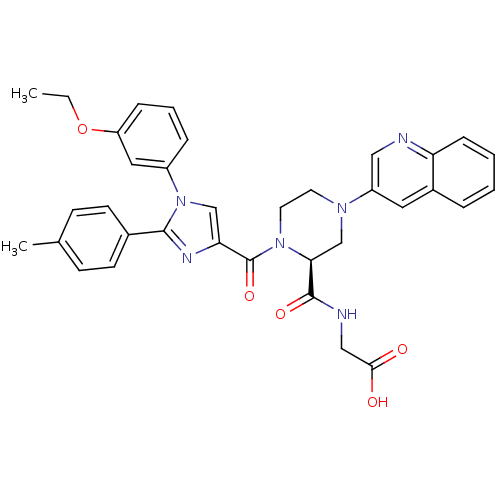
(2-((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H34N6O5/c1-3-46-28-9-6-8-26(18-28)41-21-30(38-33(41)24-13-11-23(2)12-14-24)35(45)40-16-15-39(22-31(40)34(44)37-20-32(42)43)27-17-25-7-4-5-10-29(25)36-19-27/h4-14,17-19,21,31H,3,15-16,20,22H2,1-2H3,(H,37,44)(H,42,43)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263230
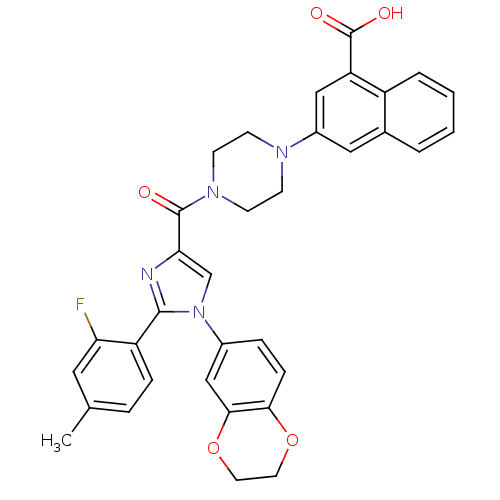
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(2...)Show SMILES Cc1ccc(-c2nc(cn2-c2ccc3OCCOc3c2)C(=O)N2CCN(CC2)c2cc(C(O)=O)c3ccccc3c2)c(F)c1 Show InChI InChI=1S/C34H29FN4O5/c1-21-6-8-26(28(35)16-21)32-36-29(20-39(32)23-7-9-30-31(19-23)44-15-14-43-30)33(40)38-12-10-37(11-13-38)24-17-22-4-2-3-5-25(22)27(18-24)34(41)42/h2-9,16-20H,10-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263229
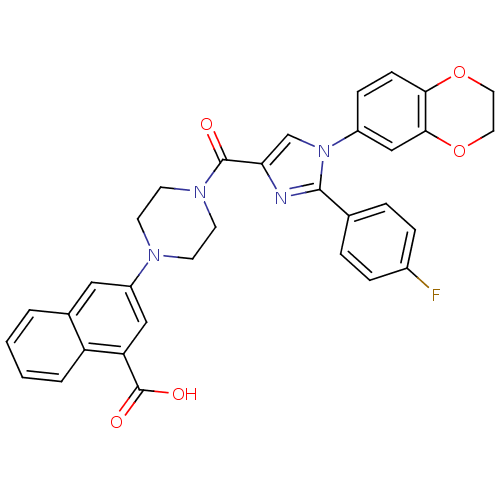
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4...)Show SMILES OC(=O)c1cc(cc2ccccc12)N1CCN(CC1)C(=O)c1cn(c(n1)-c1ccc(F)cc1)-c1ccc2OCCOc2c1 Show InChI InChI=1S/C33H27FN4O5/c34-23-7-5-21(6-8-23)31-35-28(20-38(31)24-9-10-29-30(19-24)43-16-15-42-29)32(39)37-13-11-36(12-14-37)25-17-22-3-1-2-4-26(22)27(18-25)33(40)41/h1-10,17-20H,11-16H2,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343722
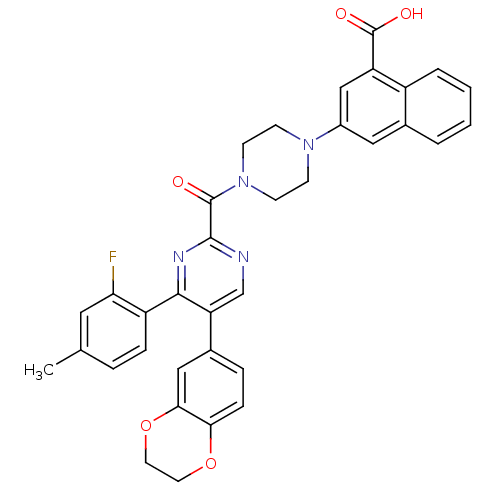
(3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...)Show SMILES Cc1ccc(c(F)c1)-c1nc(ncc1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C35H29FN4O5/c1-21-6-8-26(29(36)16-21)32-28(23-7-9-30-31(18-23)45-15-14-44-30)20-37-33(38-32)34(41)40-12-10-39(11-13-40)24-17-22-4-2-3-5-25(22)27(19-24)35(42)43/h2-9,16-20H,10-15H2,1H3,(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245195
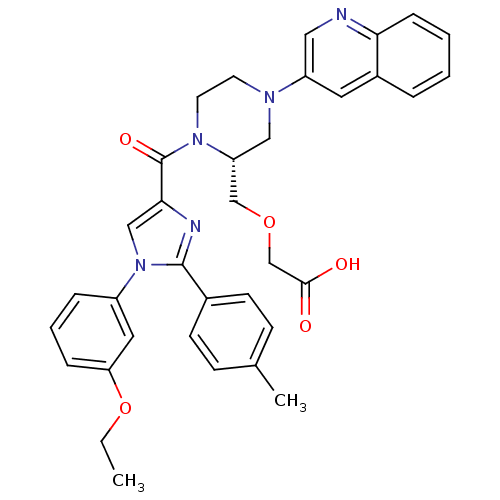
(2-(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245188
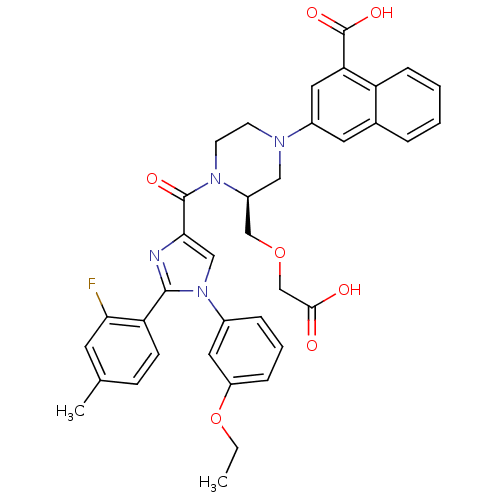
(3-((S)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H35FN4O7/c1-3-49-28-9-6-8-25(17-28)42-20-33(39-35(42)30-12-11-23(2)15-32(30)38)36(45)41-14-13-40(19-27(41)21-48-22-34(43)44)26-16-24-7-4-5-10-29(24)31(18-26)37(46)47/h4-12,15-18,20,27H,3,13-14,19,21-22H2,1-2H3,(H,43,44)(H,46,47)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245201
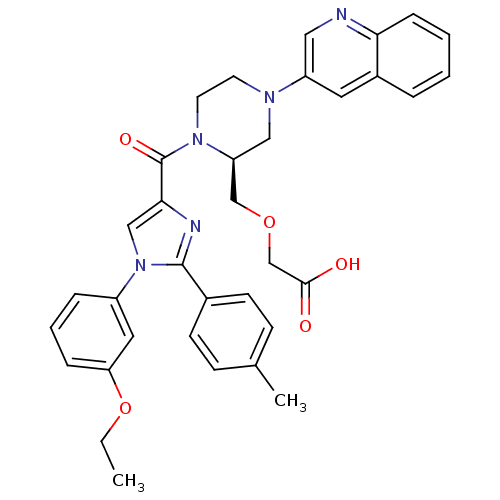
(3-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245189

(3-((R)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36FN5O5/c1-4-48-29-10-7-9-26(18-29)43-22-34(40-35(43)31-13-12-23(2)16-33(31)38)36(45)42-15-14-41(21-28(42)20-39-24(3)44)27-17-25-8-5-6-11-30(25)32(19-27)37(46)47/h5-13,16-19,22,28H,4,14-15,20-21H2,1-3H3,(H,39,44)(H,46,47)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245190
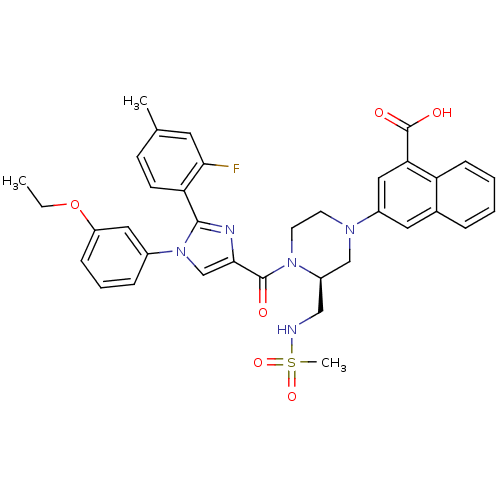
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H36FN5O6S/c1-4-48-28-10-7-9-25(18-28)42-22-33(39-34(42)30-13-12-23(2)16-32(30)37)35(43)41-15-14-40(21-27(41)20-38-49(3,46)47)26-17-24-8-5-6-11-29(24)31(19-26)36(44)45/h5-13,16-19,22,27,38H,4,14-15,20-21H2,1-3H3,(H,44,45)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263227

(3-(4-(2-(2,4-difluorophenyl)-1-(3-ethoxyphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H28F2N4O4/c1-2-43-25-8-5-7-23(18-25)39-20-30(36-31(39)27-11-10-22(34)17-29(27)35)32(40)38-14-12-37(13-15-38)24-16-21-6-3-4-9-26(21)28(19-24)33(41)42/h3-11,16-20H,2,12-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263226
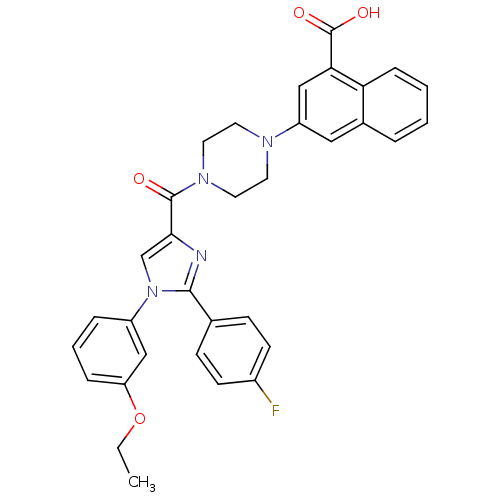
(3-(4-(1-(3-ethoxyphenyl)-2-(4-fluorophenyl)-1H-imi...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H29FN4O4/c1-2-42-27-8-5-7-25(19-27)38-21-30(35-31(38)22-10-12-24(34)13-11-22)32(39)37-16-14-36(15-17-37)26-18-23-6-3-4-9-28(23)29(20-26)33(40)41/h3-13,18-21H,2,14-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245182

(3-((S)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37N5O5/c1-4-47-31-10-7-9-28(19-31)42-23-34(39-35(42)26-14-12-24(2)13-15-26)36(44)41-17-16-40(22-30(41)21-38-25(3)43)29-18-27-8-5-6-11-32(27)33(20-29)37(45)46/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,38,43)(H,45,46)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245184
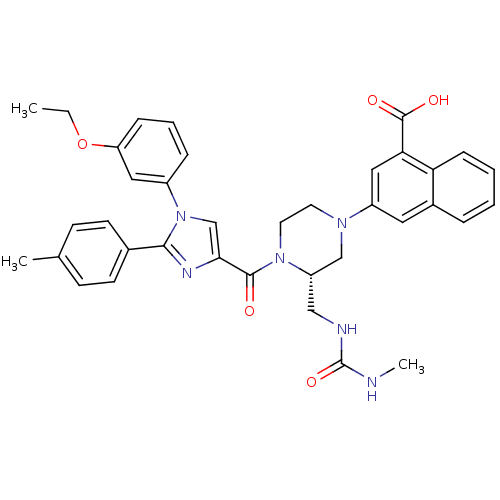
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H38N6O5/c1-4-48-30-10-7-9-27(19-30)43-23-33(40-34(43)25-14-12-24(2)13-15-25)35(44)42-17-16-41(22-29(42)21-39-37(47)38-3)28-18-26-8-5-6-11-31(26)32(20-28)36(45)46/h5-15,18-20,23,29H,4,16-17,21-22H2,1-3H3,(H,45,46)(H2,38,39,47)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245191
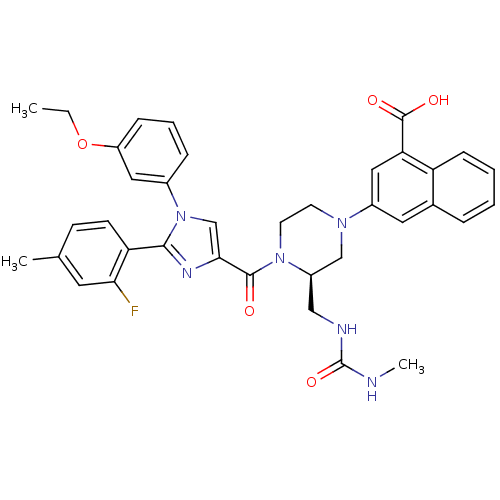
(3-((R)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37FN6O5/c1-4-49-28-10-7-9-25(18-28)44-22-33(41-34(44)30-13-12-23(2)16-32(30)38)35(45)43-15-14-42(21-27(43)20-40-37(48)39-3)26-17-24-8-5-6-11-29(24)31(19-26)36(46)47/h5-13,16-19,22,27H,4,14-15,20-21H2,1-3H3,(H,46,47)(H2,39,40,48)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245185

(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245183
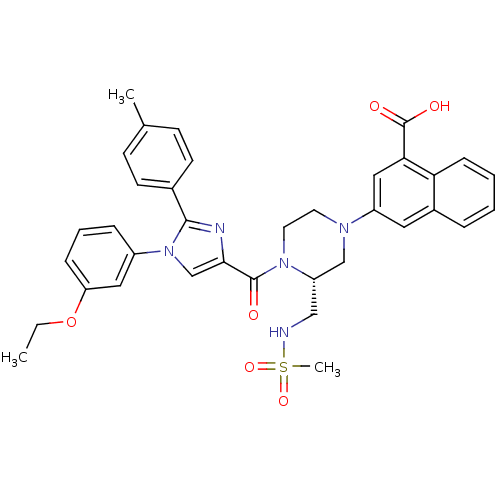
(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H37N5O6S/c1-4-47-30-10-7-9-27(19-30)41-23-33(38-34(41)25-14-12-24(2)13-15-25)35(42)40-17-16-39(22-29(40)21-37-48(3,45)46)28-18-26-8-5-6-11-31(26)32(20-28)36(43)44/h5-15,18-20,23,29,37H,4,16-17,21-22H2,1-3H3,(H,43,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180
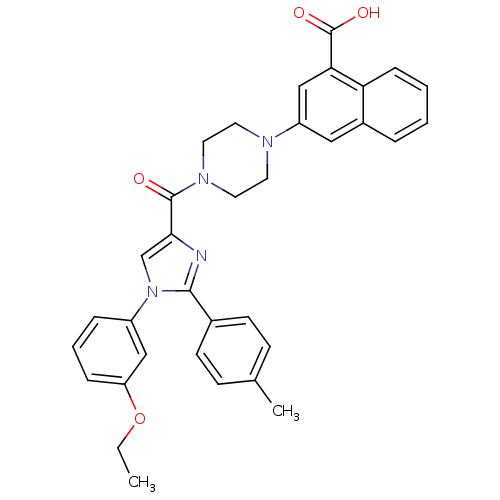
(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263225
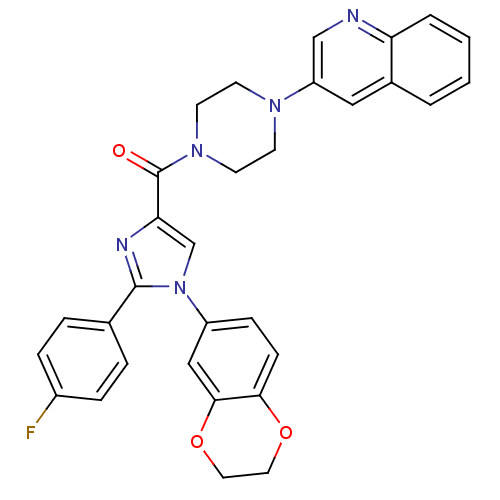
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4-fluo...)Show SMILES Fc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C31H26FN5O3/c32-23-7-5-21(6-8-23)30-34-27(20-37(30)24-9-10-28-29(18-24)40-16-15-39-28)31(38)36-13-11-35(12-14-36)25-17-22-3-1-2-4-26(22)33-19-25/h1-10,17-20H,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180

(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245181
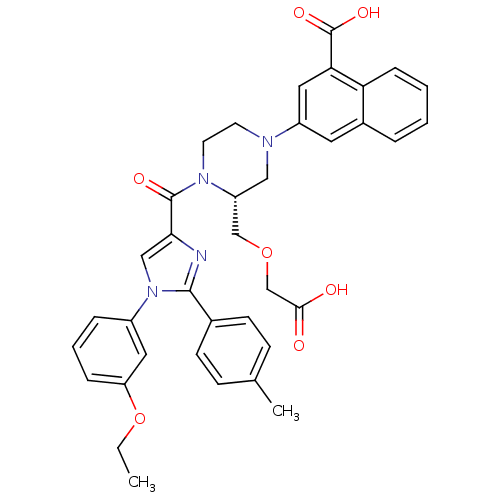
(3-((R)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36N4O7/c1-3-48-30-9-6-8-27(18-30)41-21-33(38-35(41)25-13-11-24(2)12-14-25)36(44)40-16-15-39(20-29(40)22-47-23-34(42)43)28-17-26-7-4-5-10-31(26)32(19-28)37(45)46/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,42,43)(H,45,46)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263228
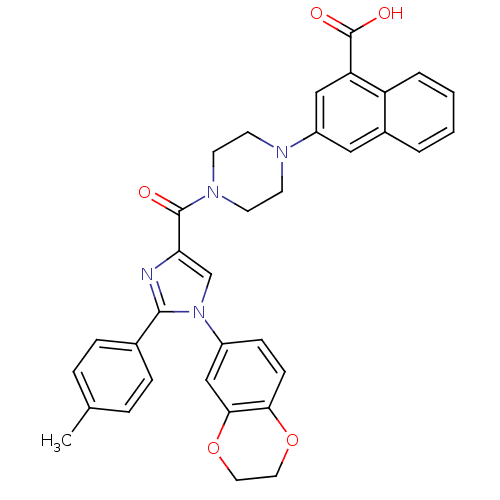
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H30N4O5/c1-22-6-8-23(9-7-22)32-35-29(21-38(32)25-10-11-30-31(20-25)43-17-16-42-30)33(39)37-14-12-36(13-15-37)26-18-24-4-2-3-5-27(24)28(19-26)34(40)41/h2-11,18-21H,12-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262862

(3-(4-(1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazole-4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H30N4O4/c1-22-10-12-23(13-11-22)31-34-30(21-37(31)25-7-5-8-27(19-25)41-2)32(38)36-16-14-35(15-17-36)26-18-24-6-3-4-9-28(24)29(20-26)33(39)40/h3-13,18-21H,14-17H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343703
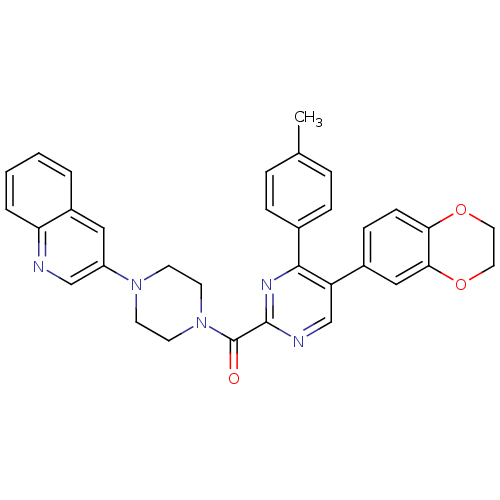
((5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(ncc1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C33H29N5O3/c1-22-6-8-23(9-7-22)31-27(24-10-11-29-30(19-24)41-17-16-40-29)21-35-32(36-31)33(39)38-14-12-37(13-15-38)26-18-25-4-2-3-5-28(25)34-20-26/h2-11,18-21H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263186
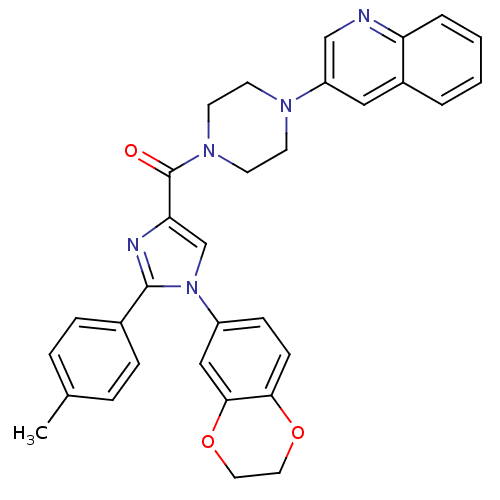
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H29N5O3/c1-22-6-8-23(9-7-22)31-34-28(21-37(31)25-10-11-29-30(19-25)40-17-16-39-29)32(38)36-14-12-35(13-15-36)26-18-24-4-2-3-5-27(24)33-20-26/h2-11,18-21H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320187
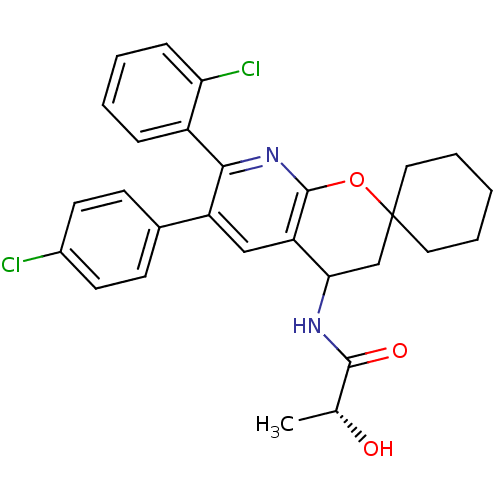
((2R)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...)Show SMILES C[C@@H](O)C(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H28Cl2N2O3/c1-17(33)26(34)31-24-16-28(13-5-2-6-14-28)35-27-22(24)15-21(18-9-11-19(29)12-10-18)25(32-27)20-7-3-4-8-23(20)30/h3-4,7-12,15,17,24,33H,2,5-6,13-14,16H2,1H3,(H,31,34)/t17-,24?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316524
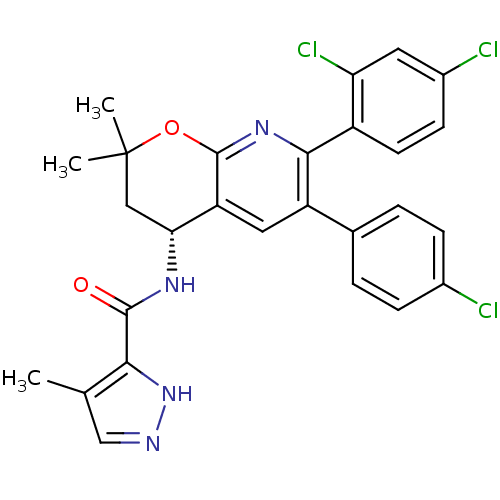
(CHEMBL1095158 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1cn[nH]c1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-13-31-34-23(14)25(35)32-22-12-27(2,3)36-26-20(22)11-19(15-4-6-16(28)7-5-15)24(33-26)18-9-8-17(29)10-21(18)30/h4-11,13,22H,12H2,1-3H3,(H,31,34)(H,32,35)/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316527
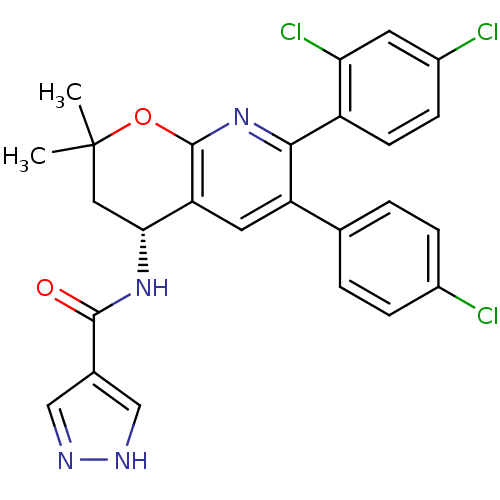
(CHEMBL1097841 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES CC1(C)C[C@@H](NC(=O)c2cn[nH]c2)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H21Cl3N4O2/c1-26(2)11-22(32-24(34)15-12-30-31-13-15)20-10-19(14-3-5-16(27)6-4-14)23(33-25(20)35-26)18-8-7-17(28)9-21(18)29/h3-10,12-13,22H,11H2,1-2H3,(H,30,31)(H,32,34)/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316529
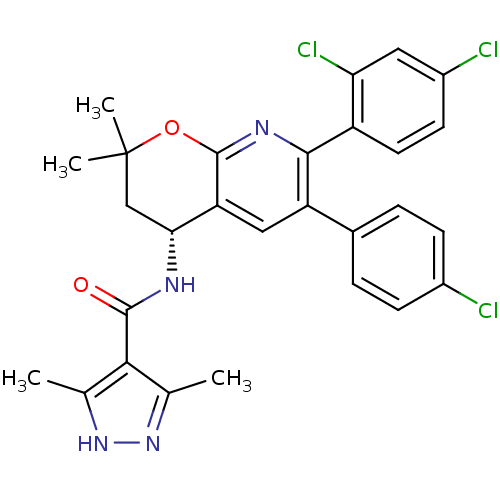
(CHEMBL1095151 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1n[nH]c(C)c1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H25Cl3N4O2/c1-14-24(15(2)35-34-14)26(36)32-23-13-28(3,4)37-27-21(23)12-20(16-5-7-17(29)8-6-16)25(33-27)19-10-9-18(30)11-22(19)31/h5-12,23H,13H2,1-4H3,(H,32,36)(H,34,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267907
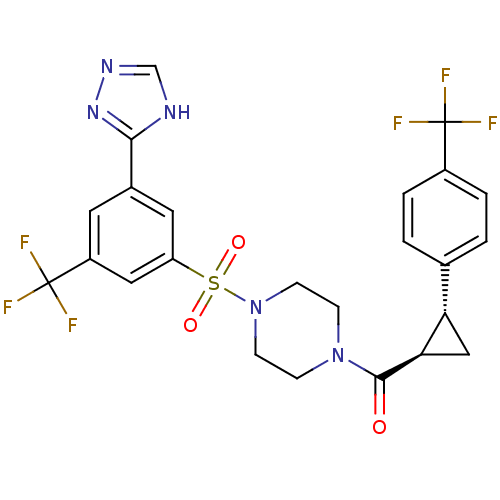
((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1nnc[nH]1 |r| Show InChI InChI=1S/C24H21F6N5O3S/c25-23(26,27)16-3-1-14(2-4-16)19-12-20(19)22(36)34-5-7-35(8-6-34)39(37,38)18-10-15(21-31-13-32-33-21)9-17(11-18)24(28,29)30/h1-4,9-11,13,19-20H,5-8,12H2,(H,31,32,33)/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245199
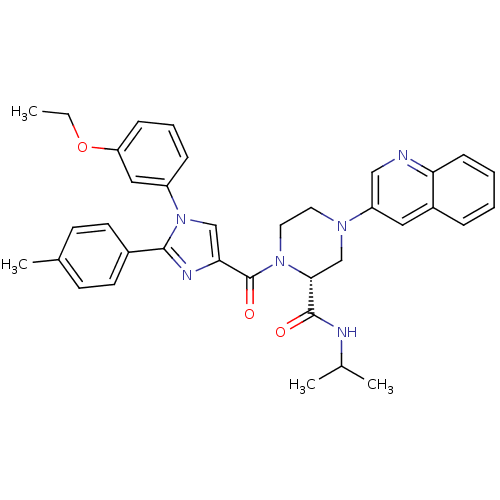
((2R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343721
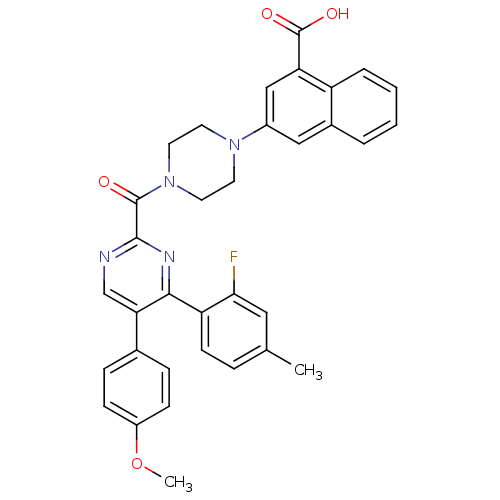
(3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...)Show SMILES COc1ccc(cc1)-c1cnc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H29FN4O4/c1-21-7-12-27(30(35)17-21)31-29(22-8-10-25(43-2)11-9-22)20-36-32(37-31)33(40)39-15-13-38(14-16-39)24-18-23-5-3-4-6-26(23)28(19-24)34(41)42/h3-12,17-20H,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263185
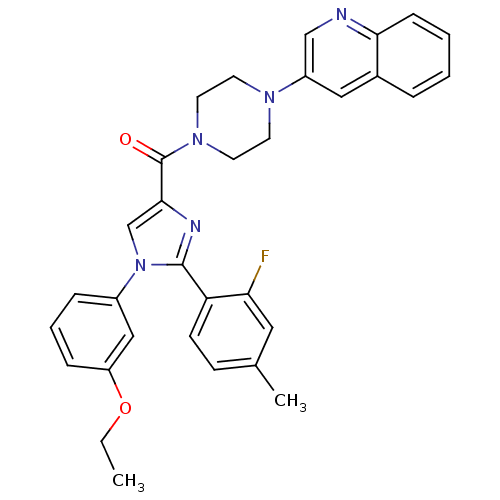
((1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H30FN5O2/c1-3-40-26-9-6-8-24(19-26)38-21-30(35-31(38)27-12-11-22(2)17-28(27)33)32(39)37-15-13-36(14-16-37)25-18-23-7-4-5-10-29(23)34-20-25/h4-12,17-21H,3,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245204

((2S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245194
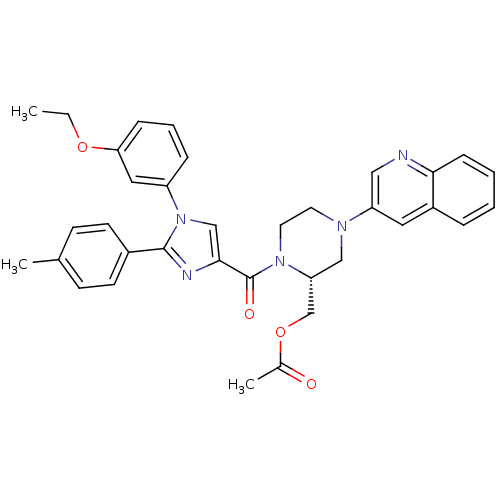
(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O4/c1-4-43-31-10-7-9-28(19-31)40-22-33(37-34(40)26-14-12-24(2)13-15-26)35(42)39-17-16-38(21-30(39)23-44-25(3)41)29-18-27-8-5-6-11-32(27)36-20-29/h5-15,18-20,22,30H,4,16-17,21,23H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245196
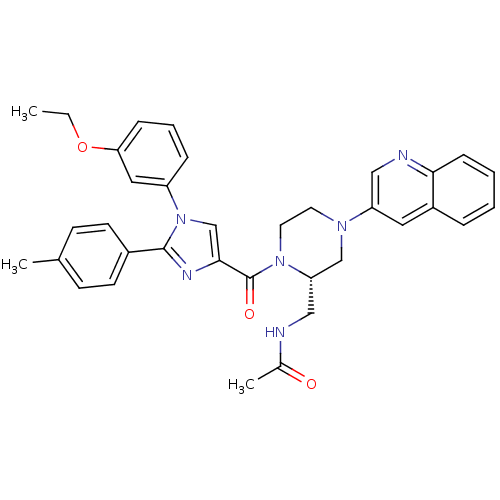
(CHEMBL450443 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343711
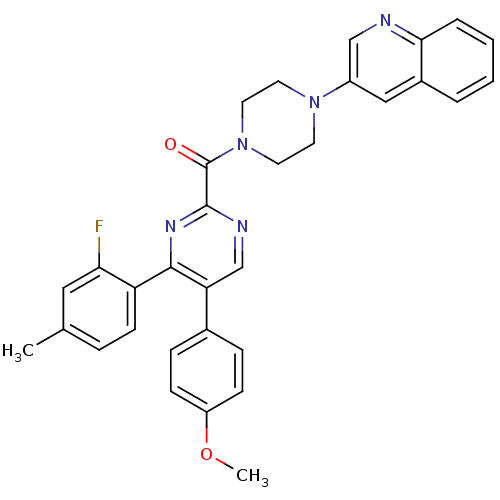
((4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphenyl)py...)Show SMILES COc1ccc(cc1)-c1cnc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H28FN5O2/c1-21-7-12-26(28(33)17-21)30-27(22-8-10-25(40-2)11-9-22)20-35-31(36-30)32(39)38-15-13-37(14-16-38)24-18-23-5-3-4-6-29(23)34-19-24/h3-12,17-20H,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50205166

(CHEMBL231636 | N-((2S,3S)-4-(4-chlorophenyl)-3-(3-...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cncc(c1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-23-13-21(15-33-16-23)27(29,30)31)24(12-18-7-9-22(28)10-8-18)20-6-4-5-19(11-20)14-32/h4-11,13,15-17,24H,12H2,1-3H3,(H,34,35)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2184-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.087
BindingDB Entry DOI: 10.7270/Q2HM584G |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50119367
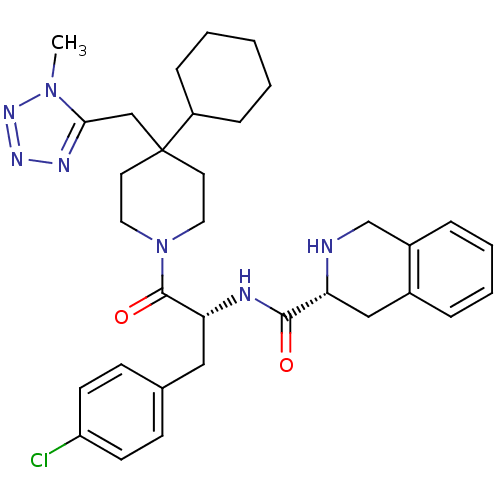
((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 Show InChI InChI=1S/C33H42ClN7O2/c1-40-30(37-38-39-40)21-33(26-9-3-2-4-10-26)15-17-41(18-16-33)32(43)29(19-23-11-13-27(34)14-12-23)36-31(42)28-20-24-7-5-6-8-25(24)22-35-28/h5-8,11-14,26,28-29,35H,2-4,9-10,15-22H2,1H3,(H,36,42)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells |
J Med Chem 45: 4589-93 (2002)
BindingDB Entry DOI: 10.7270/Q2GT5MH9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells |
J Med Chem 49: 7584-7 (2006)
Article DOI: 10.1021/jm060996+
BindingDB Entry DOI: 10.7270/Q2QN67KG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320184
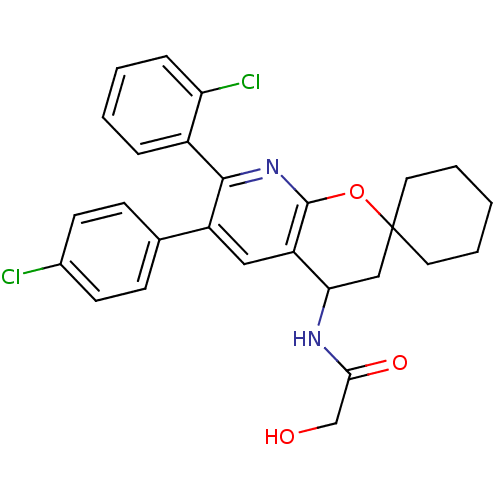
(CHEMBL1086494 | rac-N-(7'-(2-chlorophenyl)-6'-(4-c...)Show SMILES OCC(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O3/c28-18-10-8-17(9-11-18)20-14-21-23(30-24(33)16-32)15-27(12-4-1-5-13-27)34-26(21)31-25(20)19-6-2-3-7-22(19)29/h2-3,6-11,14,23,32H,1,4-5,12-13,15-16H2,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cannabinoid CB1 receptor |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at cannabinoid CB1 receptor (unknown origin) |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data