 Found 3016 hits with Last Name = 'wright' and Initial = 'a'
Found 3016 hits with Last Name = 'wright' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590042
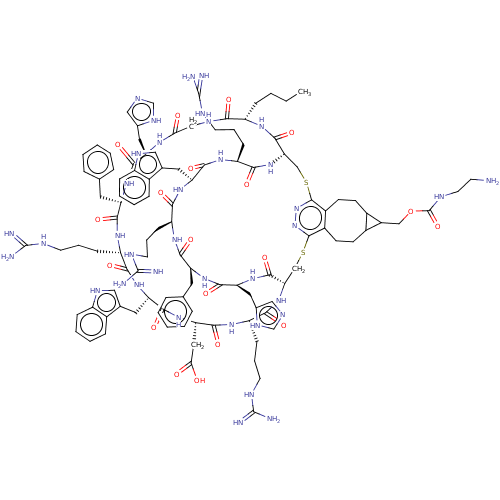
(CHEMBL5175981)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCN)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.00549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590040
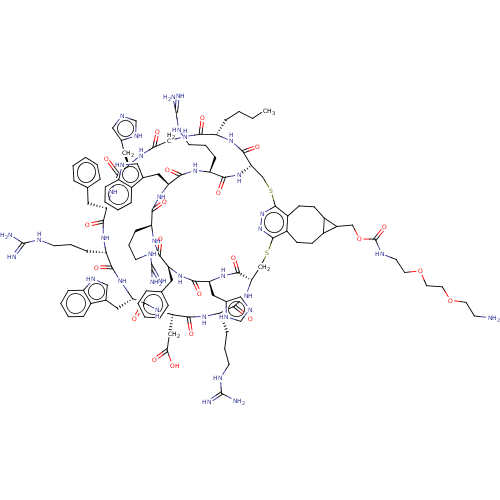
(CHEMBL5185945)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCN)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581303
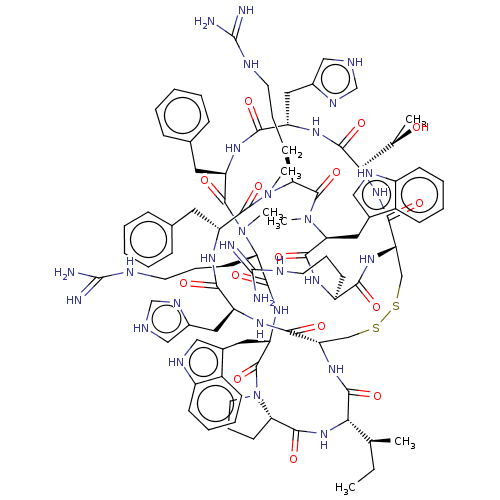
(CHEMBL5087859)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21995
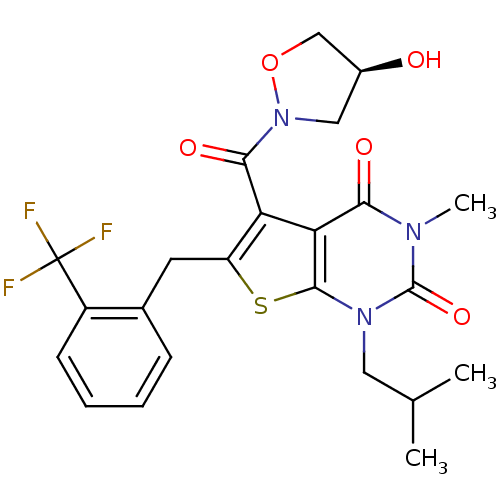
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C23H24F3N3O5S/c1-12(2)9-28-21-18(19(31)27(3)22(28)33)17(20(32)29-10-14(30)11-34-29)16(35-21)8-13-6-4-5-7-15(13)23(24,25)26/h4-7,12,14,30H,8-11H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22000
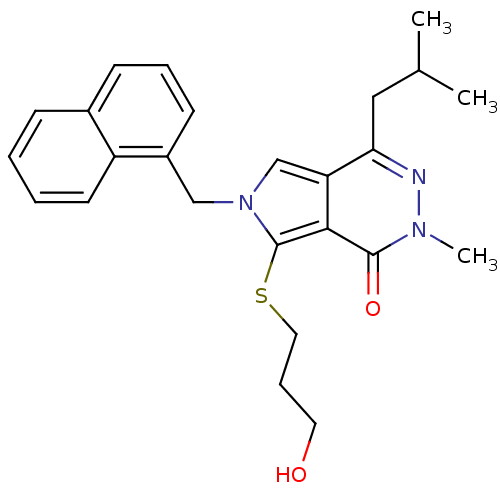
(7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...)Show SMILES CC(C)Cc1nn(C)c(=O)c2c(SCCCO)n(Cc3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H29N3O2S/c1-17(2)14-22-21-16-28(15-19-10-6-9-18-8-4-5-11-20(18)19)25(31-13-7-12-29)23(21)24(30)27(3)26-22/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R LBD expressed in HEK293 cell membranes incubated for 16 to 23 hrs in dark by scintillation proxi... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581298
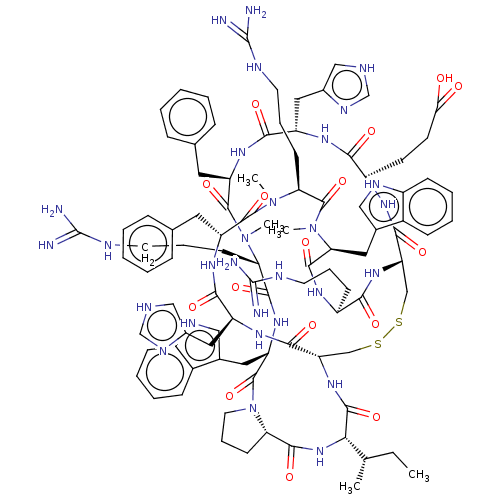
(CHEMBL5094168)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50202406
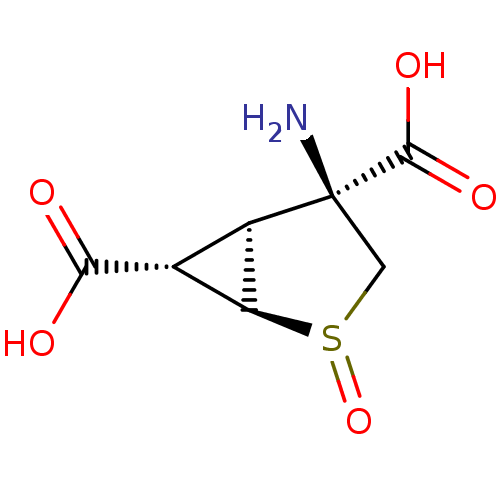
((+)-(1R,2R,4S,5S,6S)-4-amino-2-thiabicyclo[3.1.0]h...)Show SMILES N[C@]1(CS(=O)[C@H]2[C@@H]([C@@H]12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C7H9NO5S/c8-7(6(11)12)1-14(13)4-2(3(4)7)5(9)10/h2-4H,1,8H2,(H,9,10)(H,11,12)/t2-,3-,4+,7+,14?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY341495 from human recombinant mGluR3 in RGT cells |
J Med Chem 50: 233-40 (2007)
Article DOI: 10.1021/jm060917u
BindingDB Entry DOI: 10.7270/Q21Z442N |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50202406

((+)-(1R,2R,4S,5S,6S)-4-amino-2-thiabicyclo[3.1.0]h...)Show SMILES N[C@]1(CS(=O)[C@H]2[C@@H]([C@@H]12)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C7H9NO5S/c8-7(6(11)12)1-14(13)4-2(3(4)7)5(9)10/h2-4H,1,8H2,(H,9,10)(H,11,12)/t2-,3-,4+,7+,14?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]LY341495 from human recombinant mGluR3 in RGT cells |
J Med Chem 50: 233-40 (2007)
Article DOI: 10.1021/jm060917u
BindingDB Entry DOI: 10.7270/Q21Z442N |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530711
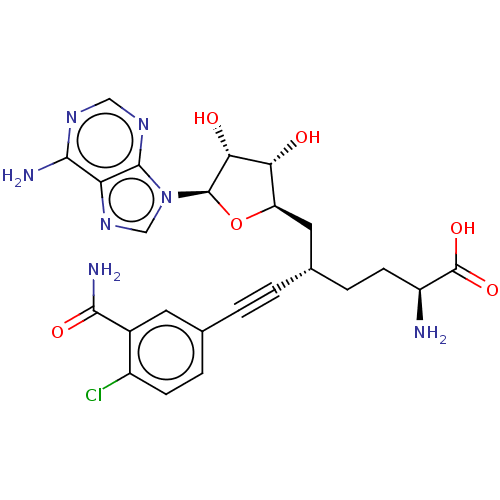
(CHEMBL4553052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(Cl)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26ClN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530711

(CHEMBL4553052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(Cl)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26ClN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530731
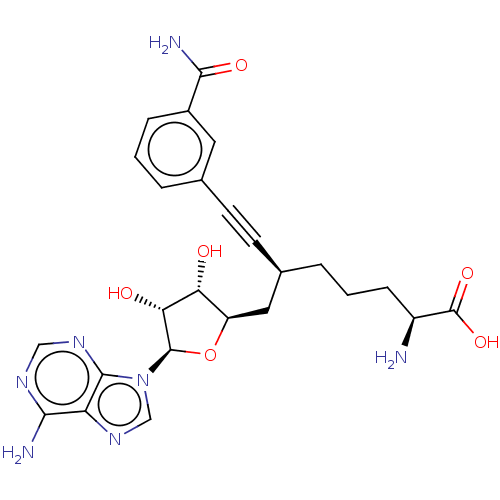
(CHEMBL4580446)Show SMILES N[C@@H](CCC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C25H29N7O6/c26-16(25(36)37)6-2-4-14(8-7-13-3-1-5-15(9-13)22(28)35)10-17-19(33)20(34)24(38-17)32-12-31-18-21(27)29-11-30-23(18)32/h1,3,5,9,11-12,14,16-17,19-20,24,33-34H,2,4,6,10,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16+,17-,19-,20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530731

(CHEMBL4580446)Show SMILES N[C@@H](CCC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C25H29N7O6/c26-16(25(36)37)6-2-4-14(8-7-13-3-1-5-15(9-13)22(28)35)10-17-19(33)20(34)24(38-17)32-12-31-18-21(27)29-11-30-23(18)32/h1,3,5,9,11-12,14,16-17,19-20,24,33-34H,2,4,6,10,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16+,17-,19-,20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590031
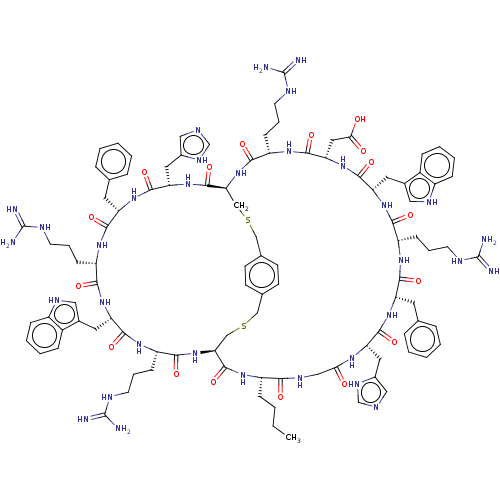
(CHEMBL5203986)Show SMILES [H][C@]12CSCc3ccc(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)cc3 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22001
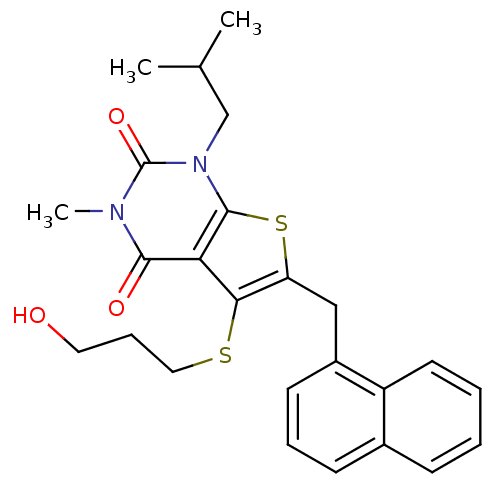
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H28N2O3S2/c1-16(2)15-27-24-21(23(29)26(3)25(27)30)22(31-13-7-12-28)20(32-24)14-18-10-6-9-17-8-4-5-11-19(17)18/h4-6,8-11,16,28H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22001

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H28N2O3S2/c1-16(2)15-27-24-21(23(29)26(3)25(27)30)22(31-13-7-12-28)20(32-24)14-18-10-6-9-17-8-4-5-11-19(17)18/h4-6,8-11,16,28H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21992
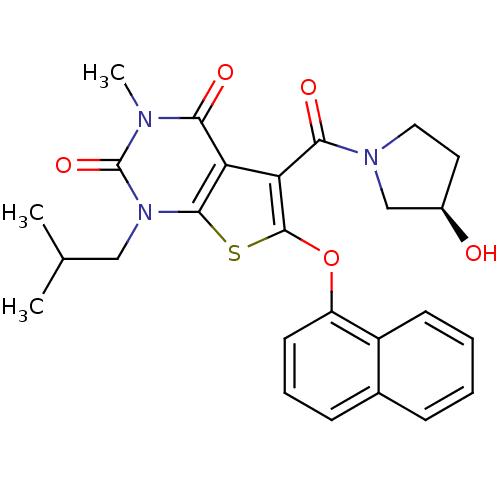
(5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...)Show SMILES CC(C)Cn1c2sc(Oc3cccc4ccccc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H27N3O5S/c1-15(2)13-29-24-20(22(31)27(3)26(29)33)21(23(32)28-12-11-17(30)14-28)25(35-24)34-19-10-6-8-16-7-4-5-9-18(16)19/h4-10,15,17,30H,11-14H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21986

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2cn(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H29N3O3S/c1-17(2)14-28-21-16-27(15-19-10-6-9-18-8-4-5-11-20(18)19)24(32-13-7-12-29)22(21)23(30)26(3)25(28)31/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21986

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2cn(Cc3cccc4ccccc34)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C25H29N3O3S/c1-17(2)14-28-21-16-27(15-19-10-6-9-18-8-4-5-11-20(18)19)24(32-13-7-12-29)22(21)23(30)26(3)25(28)31/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120502

(2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...)Show SMILES CC(C)(C)N1N=C2CCN(CC2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccc(F)c(F)c1)NC(=O)C(C)(C)N |t:5| Show InChI InChI=1S/C31H39F2N5O4/c1-29(2,3)38-28(41)31(16-20-9-7-6-8-10-20)19-37(14-13-25(31)36-38)26(39)24(35-27(40)30(4,5)34)18-42-17-21-11-12-22(32)23(33)15-21/h6-12,15,24H,13-14,16-19,34H2,1-5H3,(H,35,40)/t24-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22002
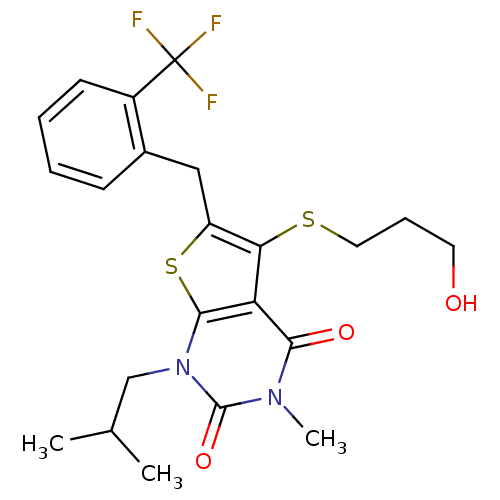
(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H25F3N2O3S2/c1-13(2)12-27-20-17(19(29)26(3)21(27)30)18(31-10-6-9-28)16(32-20)11-14-7-4-5-8-15(14)22(23,24)25/h4-5,7-8,13,28H,6,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22002

(5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...)Show SMILES CC(C)Cn1c2sc(Cc3ccccc3C(F)(F)F)c(SCCCO)c2c(=O)n(C)c1=O Show InChI InChI=1S/C22H25F3N2O3S2/c1-13(2)12-27-20-17(19(29)26(3)21(27)30)18(31-10-6-9-28)16(32-20)11-14-7-4-5-8-15(14)22(23,24)25/h4-5,7-8,13,28H,6,9-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590039
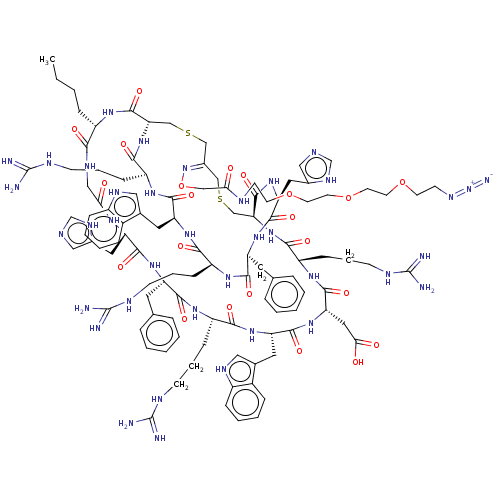
(CHEMBL5185775)Show SMILES [H][C@]12CSC\C(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)=N\OCC(=O)NCCOCCOCCOCCN=[N+]=[N-] |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590030
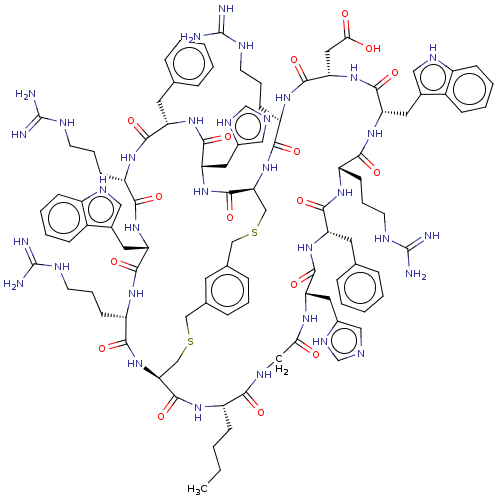
(CHEMBL5175487)Show SMILES [H][C@]12CSCc3cccc(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590047
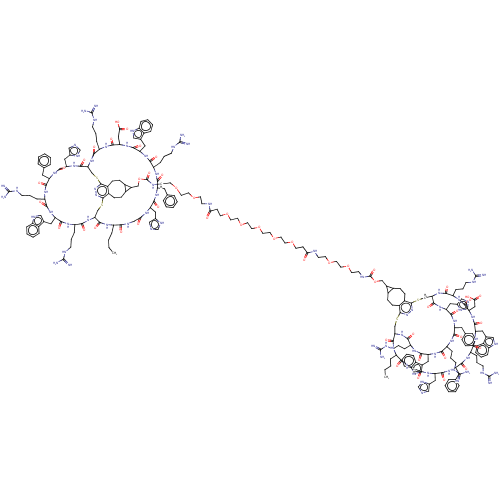
(CHEMBL5176443)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCNC(=O)CCOCCOCCOCCOCCOCCC(=O)NCCOCCOCCNC(=O)OCC4C5CCc6c(CCC45)c4SC[C@]5([H])NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc7c[nH]c8ccccc78)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc7ccccc7)NC(=O)[C@H](Cc7cnc[nH]7)NC(=O)CNC(=O)[C@H](CCCC)NC(=O)[C@]([H])(CSc6nn4)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4c[nH]c6ccccc46)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC5=O)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21996
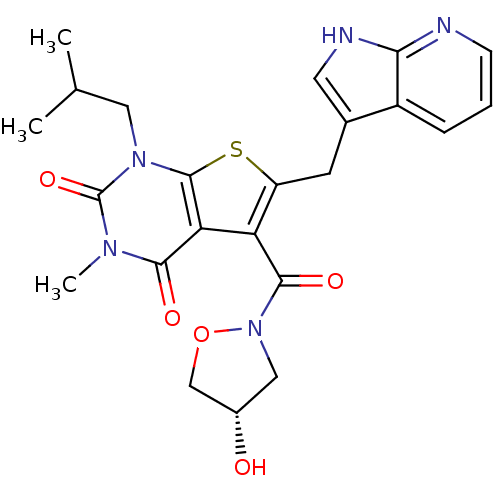
(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3c[nH]c4ncccc34)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C23H25N5O5S/c1-12(2)9-27-22-18(20(30)26(3)23(27)32)17(21(31)28-10-14(29)11-33-28)16(34-22)7-13-8-25-19-15(13)5-4-6-24-19/h4-6,8,12,14,29H,7,9-11H2,1-3H3,(H,24,25)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120504
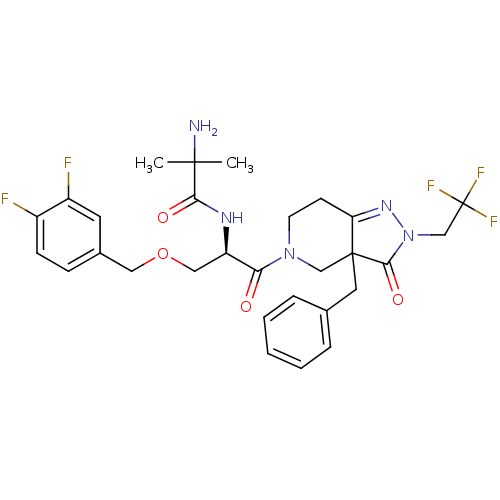
(2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccc(F)c(F)c1)C(=O)N1CCC2=NN(CC(F)(F)F)C(=O)C2(Cc2ccccc2)C1 |t:25| Show InChI InChI=1S/C29H32F5N5O4/c1-27(2,35)25(41)36-22(15-43-14-19-8-9-20(30)21(31)12-19)24(40)38-11-10-23-28(16-38,13-18-6-4-3-5-7-18)26(42)39(37-23)17-29(32,33)34/h3-9,12,22H,10-11,13-17,35H2,1-2H3,(H,36,41)/t22-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712
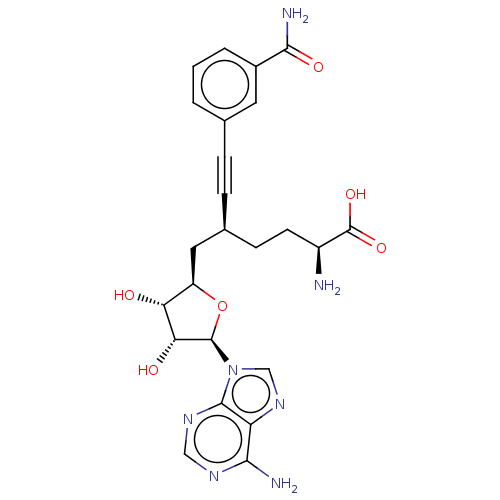
(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712

(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712

(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530712

(CHEMBL4591248)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H27N7O6/c25-15(24(35)36)7-6-13(5-4-12-2-1-3-14(8-12)21(27)34)9-16-18(32)19(33)23(37-16)31-11-30-17-20(26)28-10-29-22(17)31/h1-3,8,10-11,13,15-16,18-19,23,32-33H,6-7,9,25H2,(H2,27,34)(H,35,36)(H2,26,28,29)/t13-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21994

(5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccccc34)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C25H26N4O5S/c1-14(2)11-28-24-21(22(31)27(3)25(28)33)20(23(32)29-12-16(30)13-34-29)19(35-24)10-15-8-9-26-18-7-5-4-6-17(15)18/h4-9,14,16,30H,10-13H2,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.520 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590041
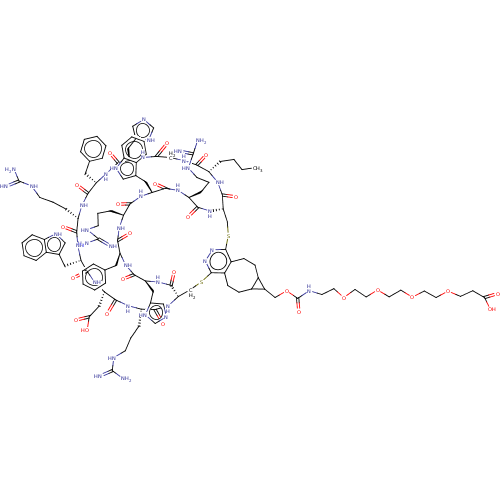
(CHEMBL5207936)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCOCCOCCC(O)=O)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590042

(CHEMBL5175981)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCN)C2CCc31 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590046
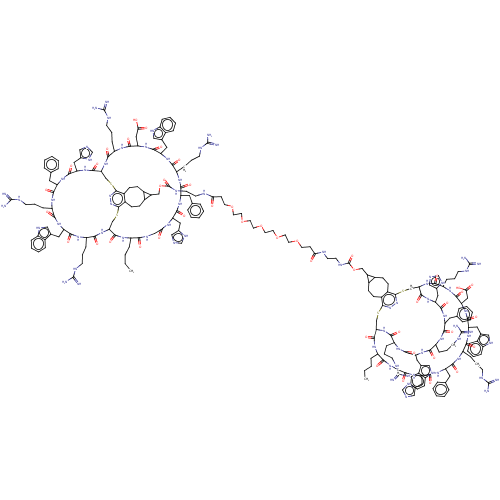
(CHEMBL5181812)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCNC(=O)CCOCCOCCOCCOCCOCCC(=O)NCCNC(=O)OCC4C5CCc6c(CCC45)c4SC[C@]5([H])NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc7c[nH]c8ccccc78)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc7ccccc7)NC(=O)[C@H](Cc7cnc[nH]7)NC(=O)CNC(=O)[C@H](CCCC)NC(=O)[C@]([H])(CSc6nn4)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4c[nH]c6ccccc46)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC5=O)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
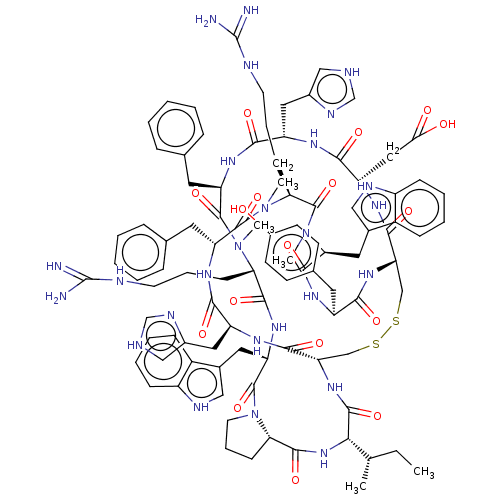
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581296
(CHEMBL5092761)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM22000

(7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...)Show SMILES CC(C)Cc1nn(C)c(=O)c2c(SCCCO)n(Cc3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H29N3O2S/c1-17(2)14-22-21-16-28(15-19-10-6-9-18-8-4-5-11-20(18)19)25(31-13-7-12-29)23(21)24(30)27(3)26-22/h4-6,8-11,16-17,29H,7,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... |
Nat Chem Biol 1: 371-6 (2005)
BindingDB Entry DOI: 10.7270/Q2MC9063 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590037
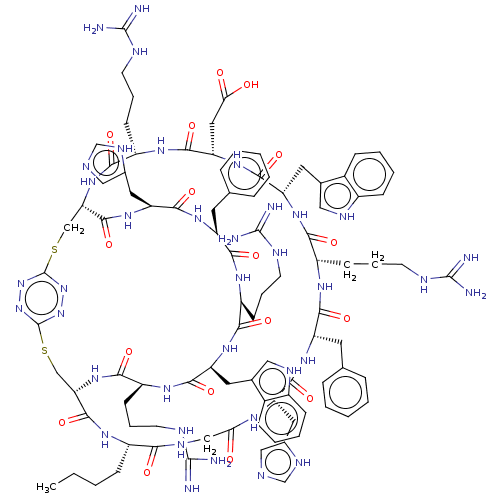
(CHEMBL5191309)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)nn3 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581313
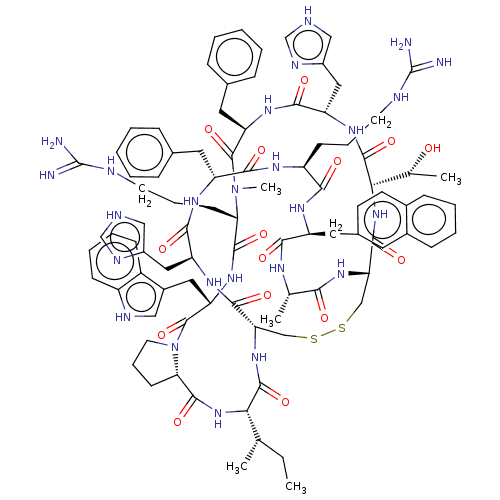
(CHEMBL5087839)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc3ccccc3c2)C(=O)N[C@@H](C)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581297
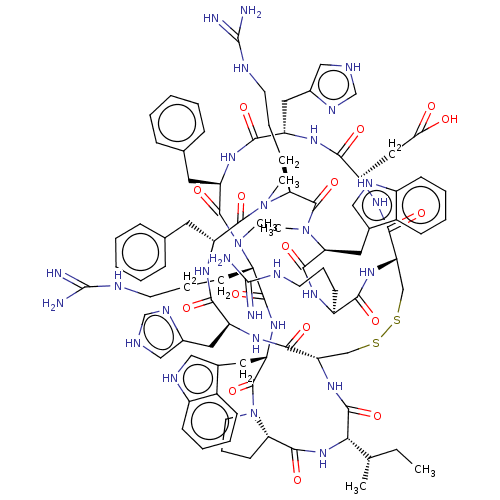
(CHEMBL5091236)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N(C)[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50590032
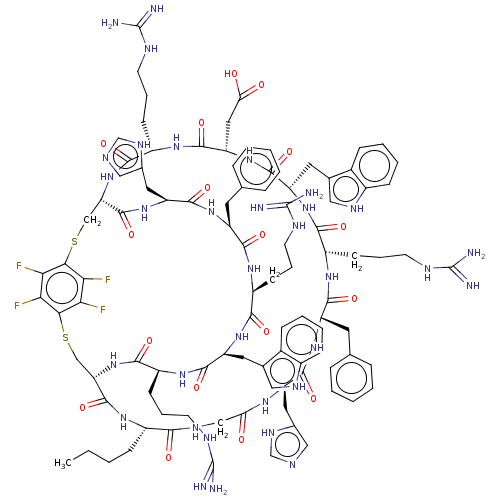
(CHEMBL5178164)Show SMILES [H][C@]12CSc3c(F)c(F)c(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c(F)c3F |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85212
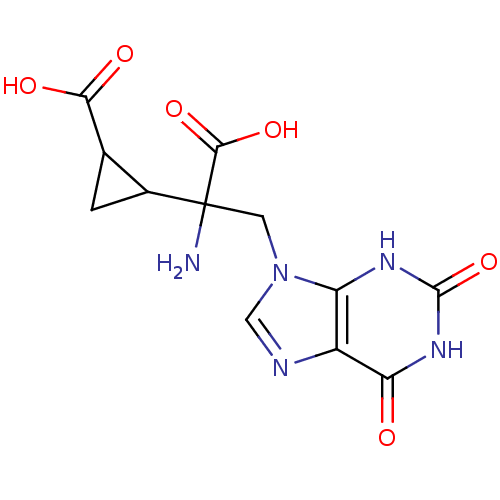
(CAS_5311260 | LY341495 | NSC_5311260)Show SMILES NC(Cn1cnc2c1[nH]c(=O)[nH]c2=O)(C1CC1C(O)=O)C(O)=O Show InChI InChI=1S/C12H13N5O6/c13-12(10(21)22,5-1-4(5)9(19)20)2-17-3-14-6-7(17)15-11(23)16-8(6)18/h3-5H,1-2,13H2,(H,19,20)(H,21,22)(H2,15,16,18,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 453-60 (2001)
BindingDB Entry DOI: 10.7270/Q2V986M2 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 1
(Homo sapiens (Human)) | BDBM21987

(6-[(6-fluoroquinolin-4-yl)methyl]-5-{[(3R)-3-hydro...)Show SMILES CC(C)Cn1c2sc(Cc3ccnc4ccc(F)cc34)c(C(=O)N3CC[C@@H](O)C3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C26H27FN4O4S/c1-14(2)12-31-25-22(23(33)29(3)26(31)35)21(24(34)30-9-7-17(32)13-30)20(36-25)10-15-6-8-28-19-5-4-16(27)11-18(15)19/h4-6,8,11,14,17,32H,7,9-10,12-13H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca
| Assay Description
Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... |
J Med Chem 50: 254-63 (2007)
Article DOI: 10.1021/jm060995h
BindingDB Entry DOI: 10.7270/Q2GT5KGF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448144
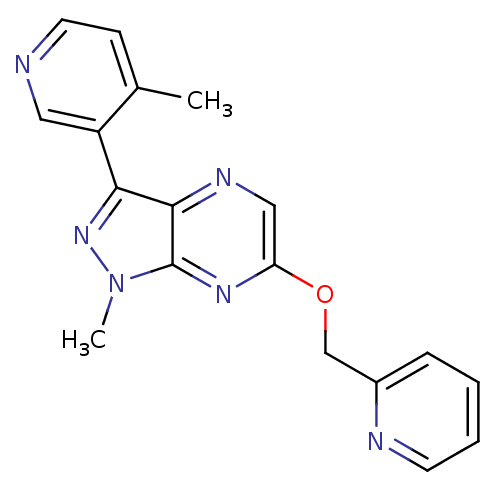
(CHEMBL3122212)Show InChI InChI=1S/C18H16N6O/c1-12-6-8-19-9-14(12)16-17-18(24(2)23-16)22-15(10-21-17)25-11-13-5-3-4-7-20-13/h3-10H,11H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50590042

(CHEMBL5175981)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCN)C2CCc31 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581311
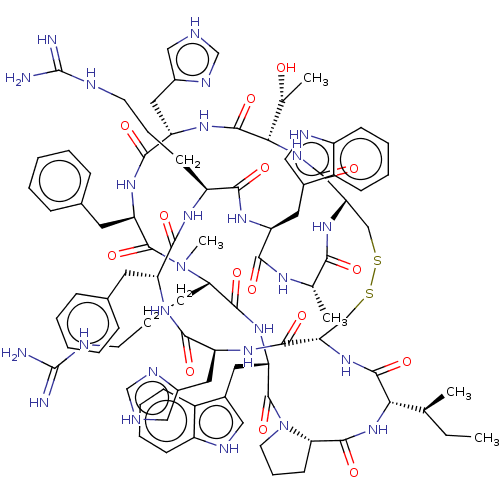
(CHEMBL5083551)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](C)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240887
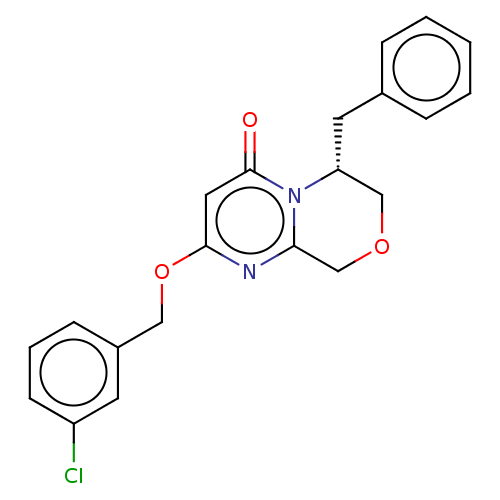
(CHEMBL4066731)Show SMILES Clc1cccc(COc2cc(=O)n3[C@H](Cc4ccccc4)COCc3n2)c1 |r| Show InChI InChI=1S/C21H19ClN2O3/c22-17-8-4-7-16(9-17)12-27-20-11-21(25)24-18(13-26-14-19(24)23-20)10-15-5-2-1-3-6-15/h1-9,11,18H,10,12-14H2/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50581306

(CHEMBL5077095)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](NC2=O)[C@@H](C)CC)C(=O)N[C@@H](Cc2c[nH]cn2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK2936E cell membrane measured after 16-23 hrs by 1450 microbeta trilux scintillat... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data