 Found 677 hits with Last Name = 'leach' and Initial = 'ag'
Found 677 hits with Last Name = 'leach' and Initial = 'ag' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095048
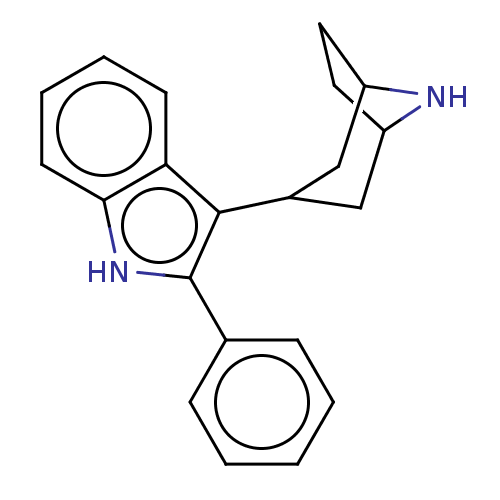
(3-[1-(8-Methyl-8-aza-bicyclo[3.2.1]oct-3-yl)-piper...)Show SMILES C1CC2CC(CC1N2)c1c([nH]c2ccccc12)-c1ccccc1 |TLB:8:4:7:0.1| Show InChI InChI=1S/C21H22N2/c1-2-6-14(7-3-1)21-20(18-8-4-5-9-19(18)23-21)15-12-16-10-11-17(13-15)22-16/h1-9,15-17,22-23H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108700
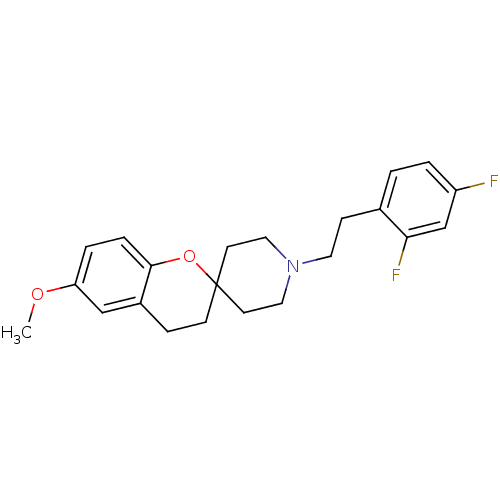
(1'-(2,4-difluorophenethyl)-6-methoxyspiro[3,4-dihy...)Show InChI InChI=1S/C22H25F2NO2/c1-26-19-4-5-21-17(14-19)6-8-22(27-21)9-12-25(13-10-22)11-7-16-2-3-18(23)15-20(16)24/h2-5,14-15H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108698
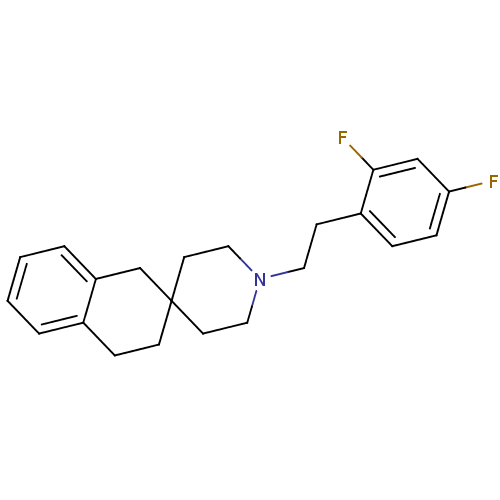
(1'-(2,4-difluorophenethyl)spiro[1,2,3,4-tetrahydro...)Show InChI InChI=1S/C22H25F2N/c23-20-6-5-18(21(24)15-20)8-12-25-13-10-22(11-14-25)9-7-17-3-1-2-4-19(17)16-22/h1-6,15H,7-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095049
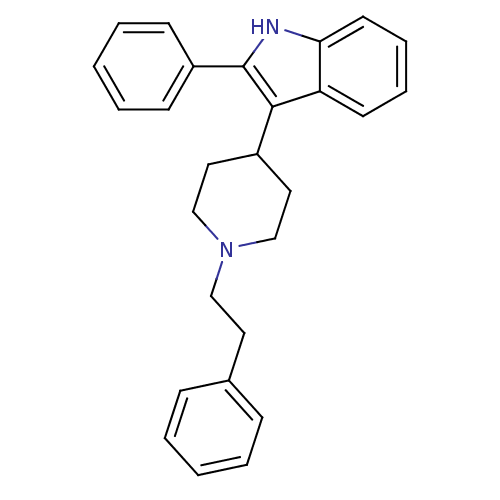
(3-(1-Phenethyl-piperidin-4-yl)-2-phenyl-1H-indole ...)Show SMILES C(Cc1ccccc1)N1CCC(CC1)c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C27H28N2/c1-3-9-21(10-4-1)15-18-29-19-16-22(17-20-29)26-24-13-7-8-14-25(24)28-27(26)23-11-5-2-6-12-23/h1-14,22,28H,15-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108697
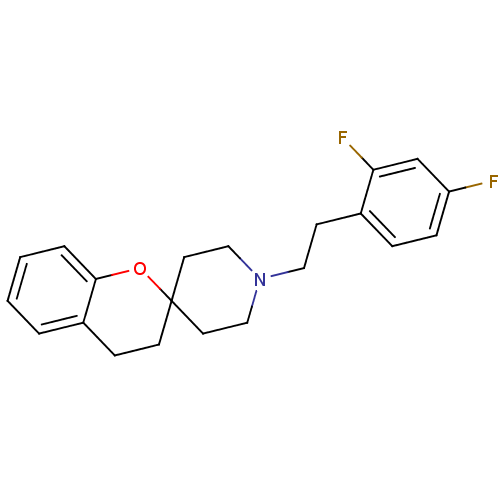
(1'-(2,4-difluorophenethyl)spiro[3,4-dihydro-2H-chr...)Show InChI InChI=1S/C21H23F2NO/c22-18-6-5-16(19(23)15-18)8-12-24-13-10-21(11-14-24)9-7-17-3-1-2-4-20(17)25-21/h1-6,15H,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099271
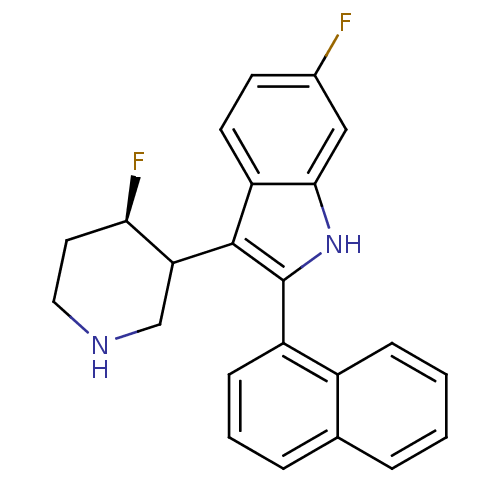
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1cccc2ccccc12 Show InChI InChI=1S/C23H20F2N2/c24-15-8-9-18-21(12-15)27-23(22(18)19-13-26-11-10-20(19)25)17-7-3-5-14-4-1-2-6-16(14)17/h1-9,12,19-20,26-27H,10-11,13H2/t19?,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108689
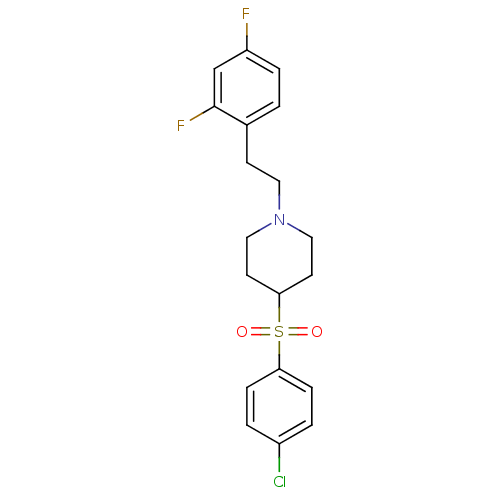
(4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C19H20ClF2NO2S/c20-15-2-5-17(6-3-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-4-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50010044
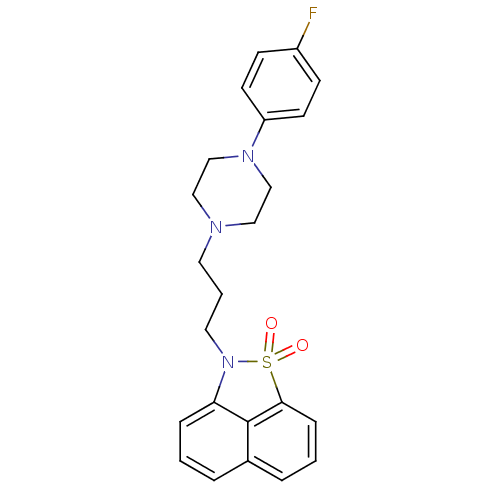
(2-{3-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-propyl}-...)Show SMILES Fc1ccc(cc1)N1CCN(CCCN2c3cccc4cccc(c34)S2(=O)=O)CC1 Show InChI InChI=1S/C23H24FN3O2S/c24-19-8-10-20(11-9-19)26-16-14-25(15-17-26)12-3-13-27-21-6-1-4-18-5-2-7-22(23(18)21)30(27,28)29/h1-2,4-11H,3,12-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099274
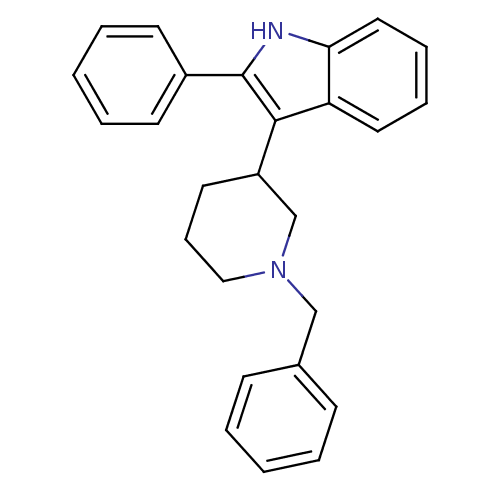
(3-(1-Benzyl-piperidin-3-yl)-2-phenyl-1H-indole | 3...)Show SMILES C(N1CCCC(C1)c1c([nH]c2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H26N2/c1-3-10-20(11-4-1)18-28-17-9-14-22(19-28)25-23-15-7-8-16-24(23)27-26(25)21-12-5-2-6-13-21/h1-8,10-13,15-16,22,27H,9,14,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099258
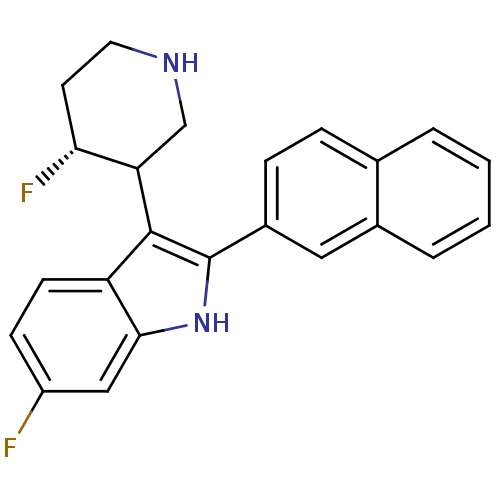
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H20F2N2/c24-17-7-8-18-21(12-17)27-23(22(18)19-13-26-10-9-20(19)25)16-6-5-14-3-1-2-4-15(14)11-16/h1-8,11-12,19-20,26-27H,9-10,13H2/t19?,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099267
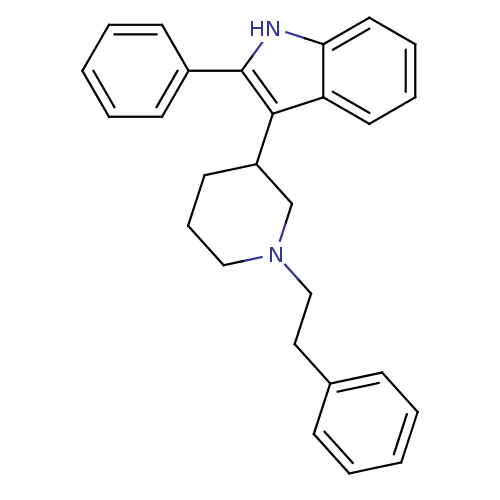
(3-(1-(2-Phenylethyl)piperidin-3-yl)-2-phenyl-1H-in...)Show SMILES C(Cc1ccccc1)N1CCCC(C1)c1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C27H28N2/c1-3-10-21(11-4-1)17-19-29-18-9-14-23(20-29)26-24-15-7-8-16-25(24)28-27(26)22-12-5-2-6-13-22/h1-8,10-13,15-16,23,28H,9,14,17-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108711
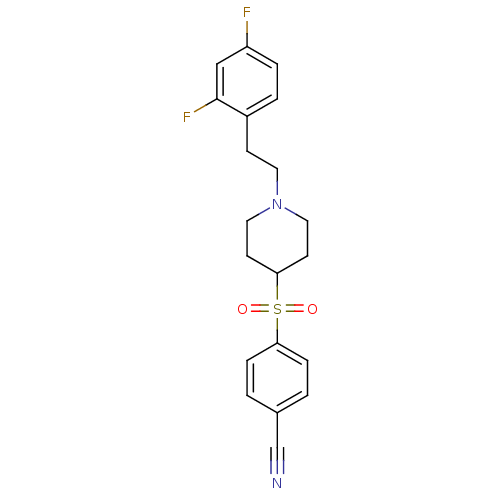
(4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccc(cc2)C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-4-3-16(20(22)13-17)7-10-24-11-8-19(9-12-24)27(25,26)18-5-1-15(14-23)2-6-18/h1-6,13,19H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108701
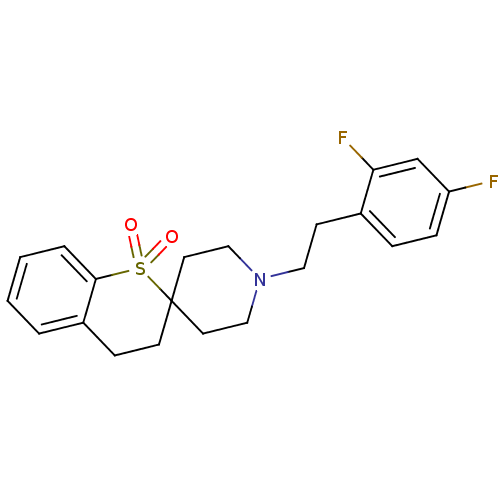
(1'-[2-(2,4-Difluorophenyl)ethyl]-3,4-dihydrospiro[...)Show SMILES Fc1ccc(CCN2CCC3(CC2)CCc2ccccc2S3(=O)=O)c(F)c1 Show InChI InChI=1S/C21H23F2NO2S/c22-18-6-5-16(19(23)15-18)8-12-24-13-10-21(11-14-24)9-7-17-3-1-2-4-20(17)27(21,25)26/h1-6,15H,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108699
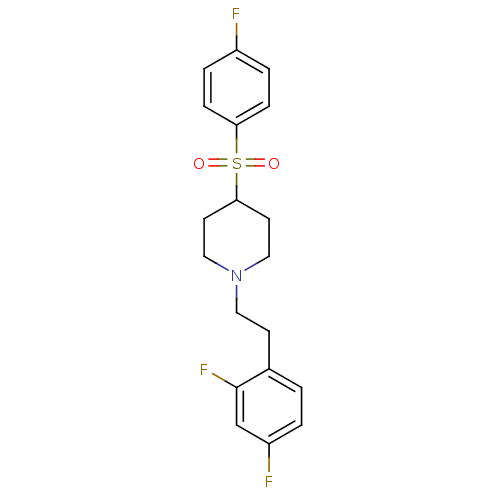
(1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H20F3NO2S/c20-15-3-5-17(6-4-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-2-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099275
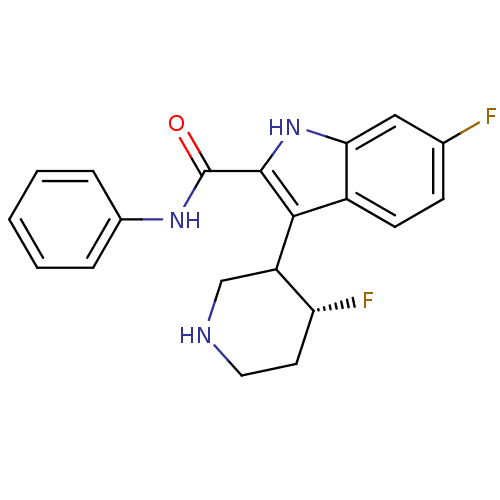
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-1H-indole-2-c...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H19F2N3O/c21-12-6-7-14-17(10-12)25-19(18(14)15-11-23-9-8-16(15)22)20(26)24-13-4-2-1-3-5-13/h1-7,10,15-16,23,25H,8-9,11H2,(H,24,26)/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108690
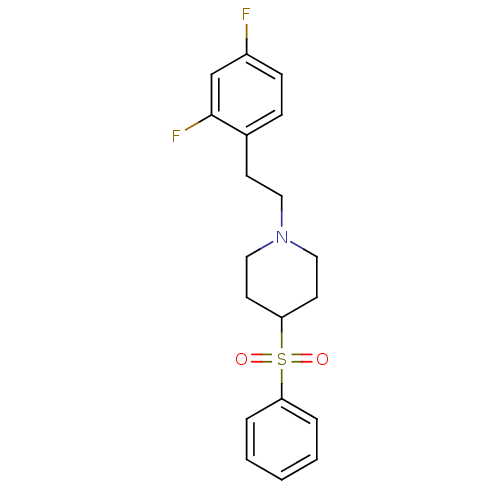
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095027
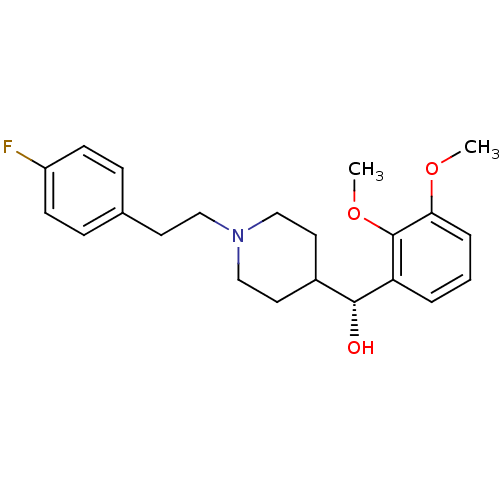
((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099260
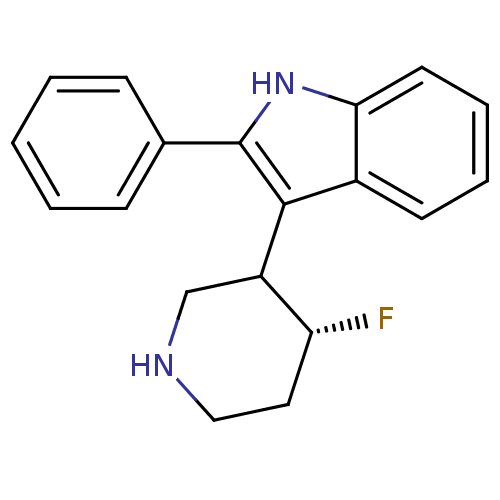
(3-(4-Fluoro-piperidin-3-yl)-2-phenyl-1H-indole | C...)Show InChI InChI=1S/C19H19FN2/c20-16-10-11-21-12-15(16)18-14-8-4-5-9-17(14)22-19(18)13-6-2-1-3-7-13/h1-9,15-16,21-22H,10-12H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50036756
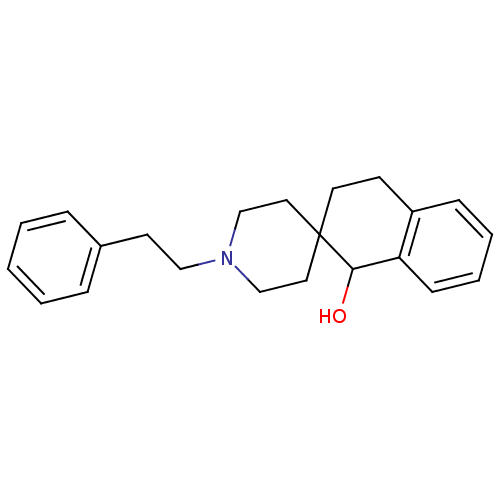
(1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...)Show InChI InChI=1S/C22H27NO/c24-21-20-9-5-4-8-19(20)10-12-22(21)13-16-23(17-14-22)15-11-18-6-2-1-3-7-18/h1-9,21,24H,10-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099255
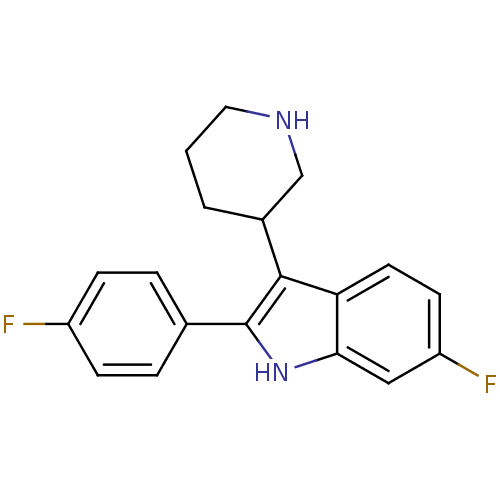
(6-Fluoro-2-(4-fluoro-phenyl)-3-piperidin-3-yl-1H-i...)Show InChI InChI=1S/C19H18F2N2/c20-14-5-3-12(4-6-14)19-18(13-2-1-9-22-11-13)16-8-7-15(21)10-17(16)23-19/h3-8,10,13,22-23H,1-2,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108706
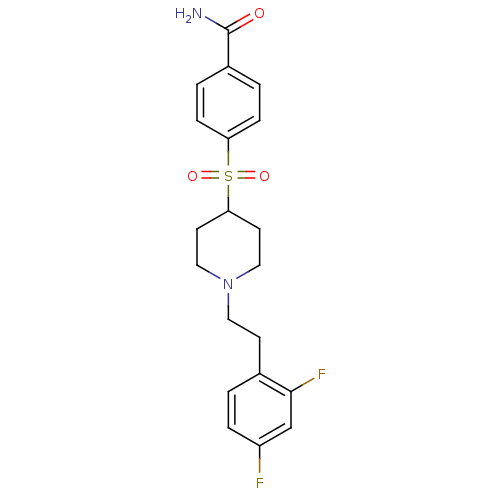
(4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES NC(=O)c1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H22F2N2O3S/c21-16-4-1-14(19(22)13-16)7-10-24-11-8-18(9-12-24)28(26,27)17-5-2-15(3-6-17)20(23)25/h1-6,13,18H,7-12H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099257
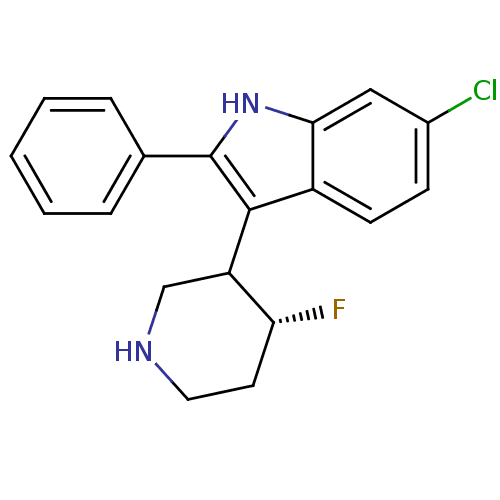
(6-Chloro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(Cl)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18ClFN2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099259
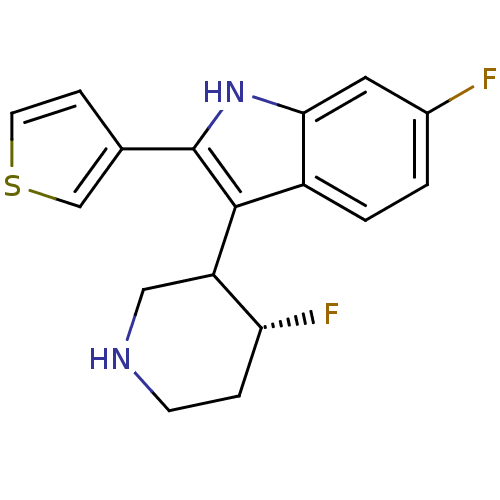
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-thiophen-3-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccsc1 Show InChI InChI=1S/C17H16F2N2S/c18-11-1-2-12-15(7-11)21-17(10-4-6-22-9-10)16(12)13-8-20-5-3-14(13)19/h1-2,4,6-7,9,13-14,20-21H,3,5,8H2/t13?,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099269
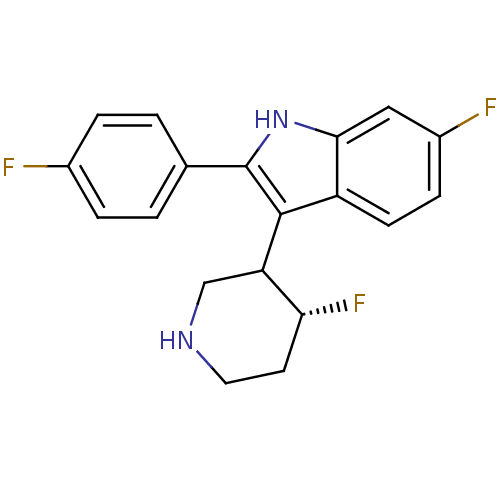
(6-Fluoro-2-(4-fluoro-phenyl)-3-(4-fluoro-piperidin...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccc(F)cc1 Show InChI InChI=1S/C19H17F3N2/c20-12-3-1-11(2-4-12)19-18(15-10-23-8-7-16(15)22)14-6-5-13(21)9-17(14)24-19/h1-6,9,15-16,23-24H,7-8,10H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108703
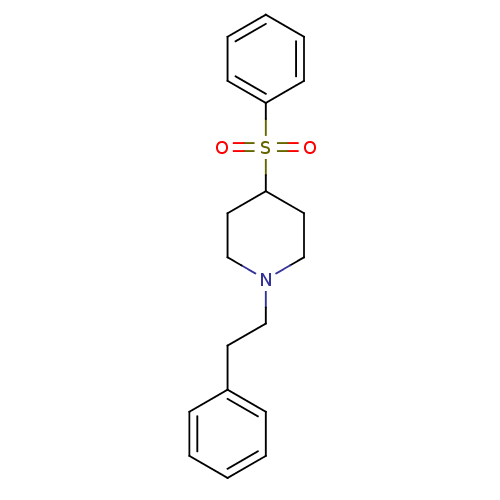
(1-(2-Phenylethyl)-4-(phenylsulfonyl)piperidine | 4...)Show InChI InChI=1S/C19H23NO2S/c21-23(22,18-9-5-2-6-10-18)19-12-15-20(16-13-19)14-11-17-7-3-1-4-8-17/h1-10,19H,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108707
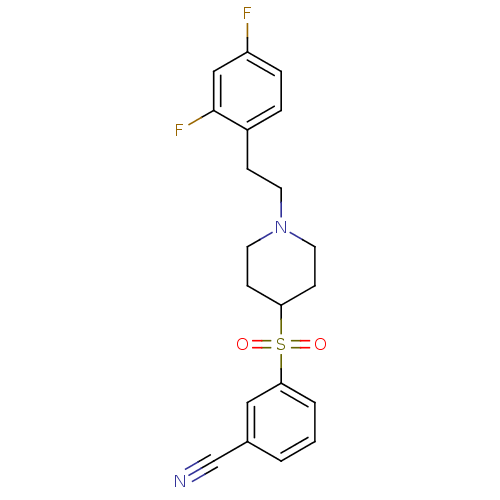
(3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2cccc(c2)C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-5-4-16(20(22)13-17)6-9-24-10-7-18(8-11-24)27(25,26)19-3-1-2-15(12-19)14-23/h1-5,12-13,18H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099270
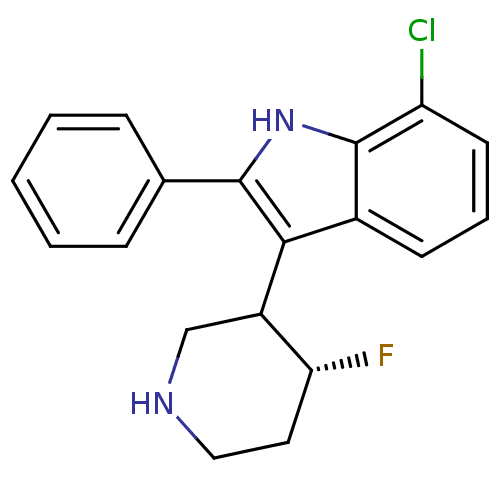
(7-Chloro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2c(Cl)cccc12)-c1ccccc1 Show InChI InChI=1S/C19H18ClFN2/c20-15-8-4-7-13-17(14-11-22-10-9-16(14)21)18(23-19(13)15)12-5-2-1-3-6-12/h1-8,14,16,22-23H,9-11H2/t14?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099276
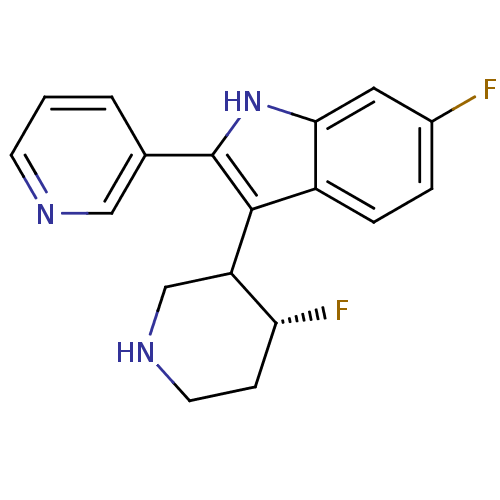
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-pyridin-3-y...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1cccnc1 Show InChI InChI=1S/C18H17F2N3/c19-12-3-4-13-16(8-12)23-18(11-2-1-6-21-9-11)17(13)14-10-22-7-5-15(14)20/h1-4,6,8-9,14-15,22-23H,5,7,10H2/t14?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108708
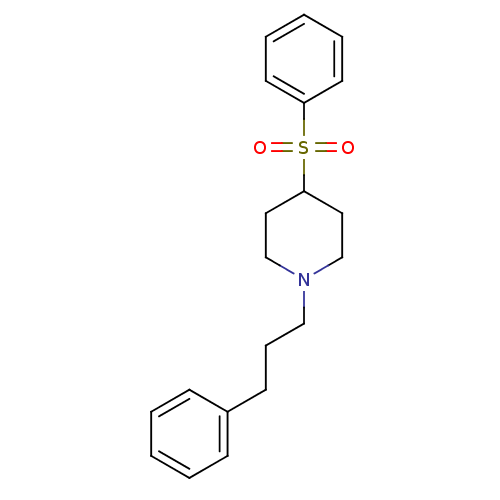
(1-(3-Phenylpropyl)-4-(phenylsulfonyl)piperidine | ...)Show InChI InChI=1S/C20H25NO2S/c22-24(23,19-11-5-2-6-12-19)20-13-16-21(17-14-20)15-7-10-18-8-3-1-4-9-18/h1-6,8-9,11-12,20H,7,10,13-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099265
(7-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2c(F)cccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-15-9-10-22-11-14(15)17-13-7-4-8-16(21)19(13)23-18(17)12-5-2-1-3-6-12/h1-8,14-15,22-23H,9-11H2/t14?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108694
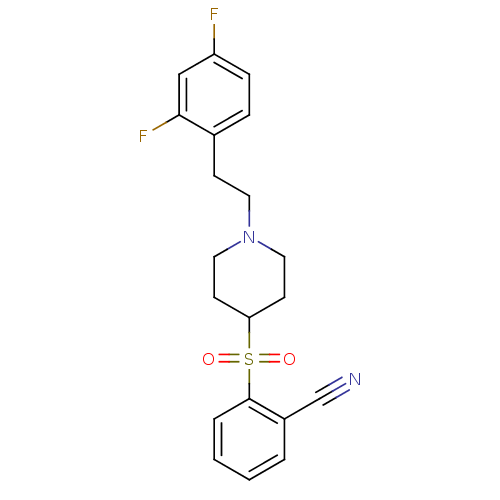
(2-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-6-5-15(19(22)13-17)7-10-24-11-8-18(9-12-24)27(25,26)20-4-2-1-3-16(20)14-23/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099273
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099262
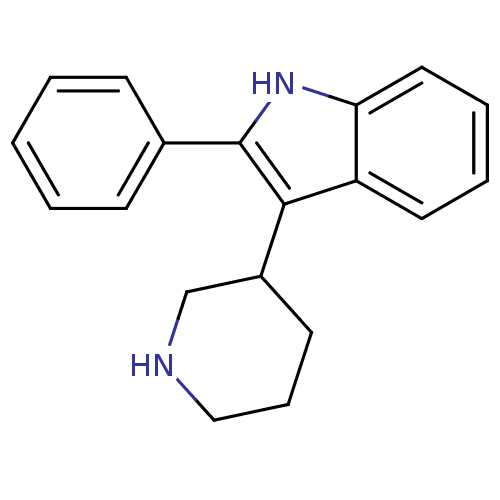
(2-Phenyl-3-piperidin-3-yl-1H-indole | 3-(Piperidin...)Show InChI InChI=1S/C19H20N2/c1-2-7-14(8-3-1)19-18(15-9-6-12-20-13-15)16-10-4-5-11-17(16)21-19/h1-5,7-8,10-11,15,20-21H,6,9,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099268
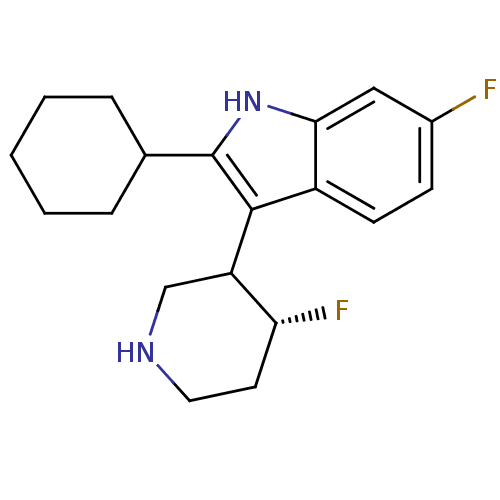
(2-Cyclohexyl-6-fluoro-3-(4-fluoro-piperidin-3-yl)-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)C1CCCCC1 Show InChI InChI=1S/C19H24F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h6-7,10,12,15-16,22-23H,1-5,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50095050
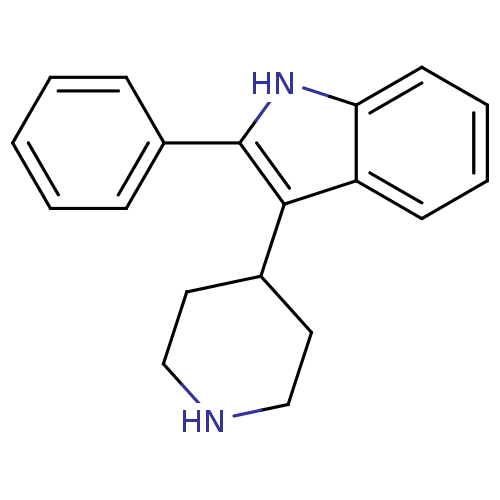
(2-Phenyl-3-piperidin-4-yl-1H-indole | 2-phenyl-3-(...)Show InChI InChI=1S/C19H20N2/c1-2-6-15(7-3-1)19-18(14-10-12-20-13-11-14)16-8-4-5-9-17(16)21-19/h1-9,14,20-21H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108705
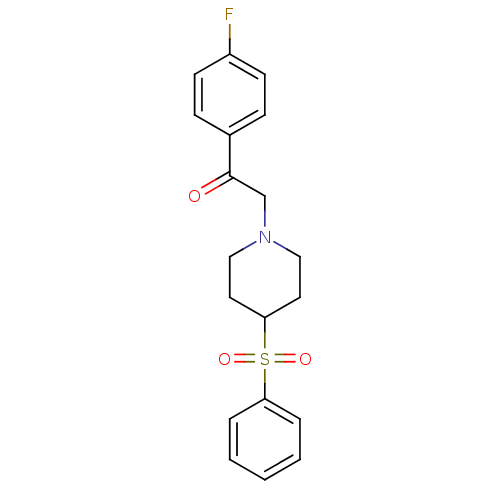
(1-(4-Fluorophenyl)-2-[4-(phenylsulfonyl)-1-piperid...)Show SMILES Fc1ccc(cc1)C(=O)CN1CCC(CC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H20FNO3S/c20-16-8-6-15(7-9-16)19(22)14-21-12-10-18(11-13-21)25(23,24)17-4-2-1-3-5-17/h1-9,18H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099263
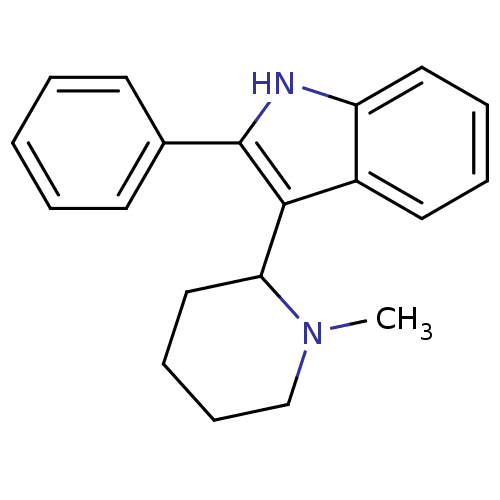
(3-(1-Methyl-piperidin-2-yl)-2-phenyl-1H-indole | 3...)Show InChI InChI=1S/C20H22N2/c1-22-14-8-7-13-18(22)19-16-11-5-6-12-17(16)21-20(19)15-9-3-2-4-10-15/h2-6,9-12,18,21H,7-8,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099254
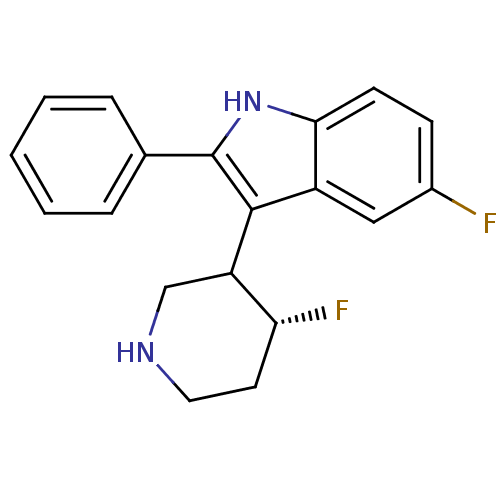
(5-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2ccc(F)cc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-17-14(10-13)18(15-11-22-9-8-16(15)21)19(23-17)12-4-2-1-3-5-12/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099266

(5-Chloro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2ccc(Cl)cc12)-c1ccccc1 Show InChI InChI=1S/C19H18ClFN2/c20-13-6-7-17-14(10-13)18(15-11-22-9-8-16(15)21)19(23-17)12-4-2-1-3-5-12/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108696
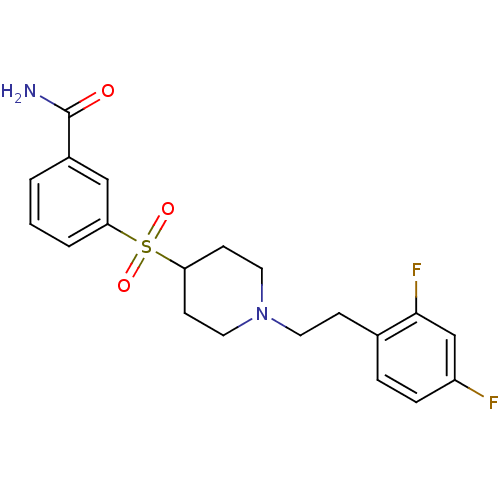
(3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES NC(=O)c1cccc(c1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H22F2N2O3S/c21-16-5-4-14(19(22)13-16)6-9-24-10-7-17(8-11-24)28(26,27)18-3-1-2-15(12-18)20(23)25/h1-5,12-13,17H,6-11H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099261
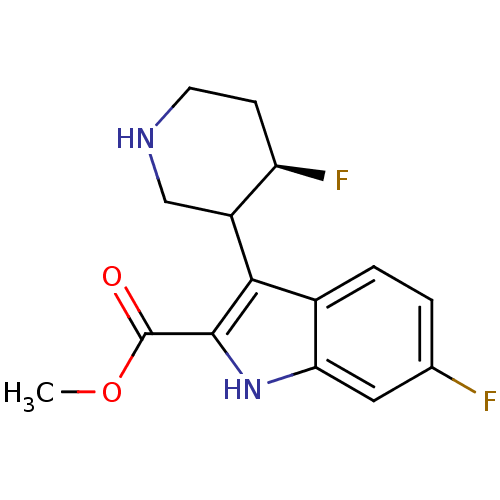
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-1H-indole-2-c...)Show InChI InChI=1S/C15H16F2N2O2/c1-21-15(20)14-13(10-7-18-5-4-11(10)17)9-3-2-8(16)6-12(9)19-14/h2-3,6,10-11,18-19H,4-5,7H2,1H3/t10?,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50099264
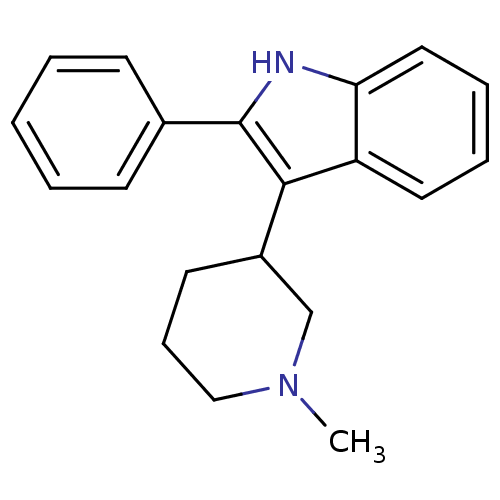
(3-(1-Methyl-piperidin-3-yl)-2-phenyl-1H-indole | 3...)Show InChI InChI=1S/C20H22N2/c1-22-13-7-10-16(14-22)19-17-11-5-6-12-18(17)21-20(19)15-8-3-2-4-9-15/h2-6,8-9,11-12,16,21H,7,10,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel |
J Med Chem 52: 4266-76 (2009)
Article DOI: 10.1021/jm900002x
BindingDB Entry DOI: 10.7270/Q2MK6DT2 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Rattus norvegicus (rat)) | BDBM50385398
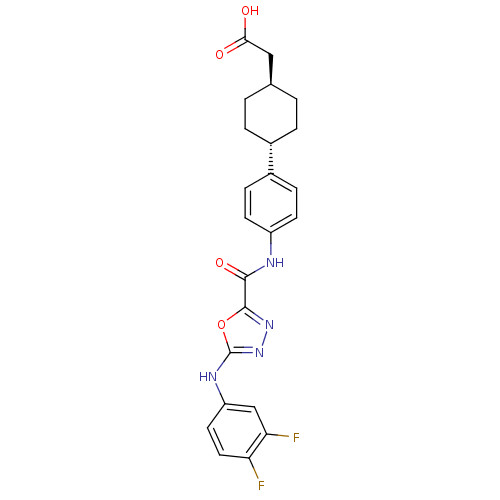
(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat DGAT1 |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1-mediated triacylglycerol synthesis in human HuTu80 cells |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293243
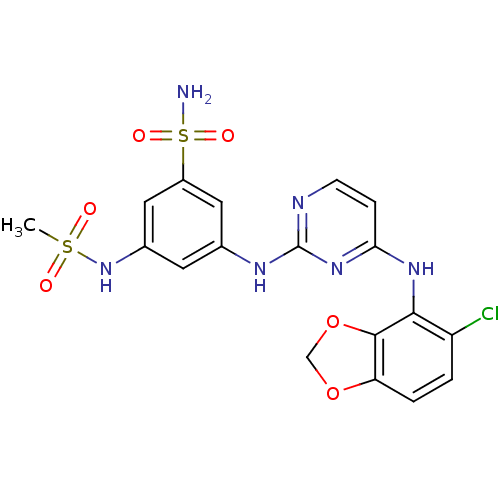
(3-(4-(5-chlorobenzo[d][1,3]dioxol-4-ylamino)pyrimi...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H17ClN6O6S2/c1-32(26,27)25-11-6-10(7-12(8-11)33(20,28)29)22-18-21-5-4-15(24-18)23-16-13(19)2-3-14-17(16)31-9-30-14/h2-8,25H,9H2,1H3,(H2,20,28,29)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400147
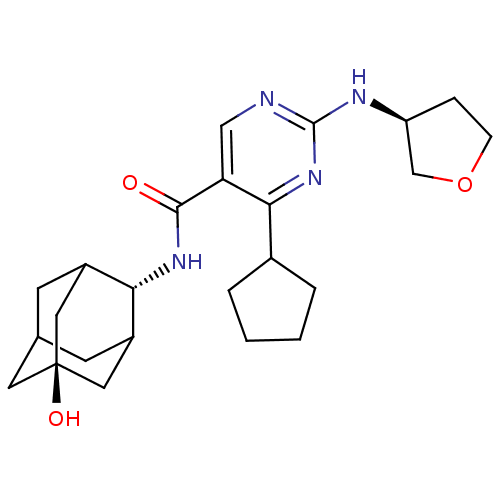
(CHEMBL2179014)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(N[C@H]4CCOC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,16.16,wD:1.0,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(33.95,-38.8,;32.42,-38.79,;31.38,-37.67,;29.96,-38.21,;29.31,-39.49,;30.34,-40.56,;31.64,-40.01,;30.35,-42.31,;29.02,-43.08,;27.68,-42.32,;27.68,-40.78,;26.35,-43.09,;25.02,-42.33,;23.69,-43.1,;23.69,-44.64,;22.35,-45.41,;21.02,-44.64,;19.61,-45.27,;18.58,-44.13,;19.35,-42.79,;20.86,-43.11,;25.02,-45.41,;26.36,-44.64,;27.69,-45.41,;27.86,-46.94,;29.37,-47.26,;30.13,-45.92,;29.1,-44.78,;31.15,-40.98,;29.98,-39.66,;32.56,-40.42,)| Show InChI InChI=1S/C24H34N4O3/c29-22(27-20-16-7-14-8-17(20)11-24(30,9-14)10-16)19-12-25-23(26-18-5-6-31-13-18)28-21(19)15-3-1-2-4-15/h12,14-18,20,30H,1-11,13H2,(H,27,29)(H,25,26,28)/t14?,16?,17?,18-,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HPLC assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293244

(CHEMBL523744 | N-(3-(4-(5-chlorobenzo[d][1,3]dioxo...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H23ClN6O5S/c1-35(30,31)28-15-10-14(11-16(12-15)29-6-8-32-9-7-29)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)34-13-33-18/h2-5,10-12,28H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247
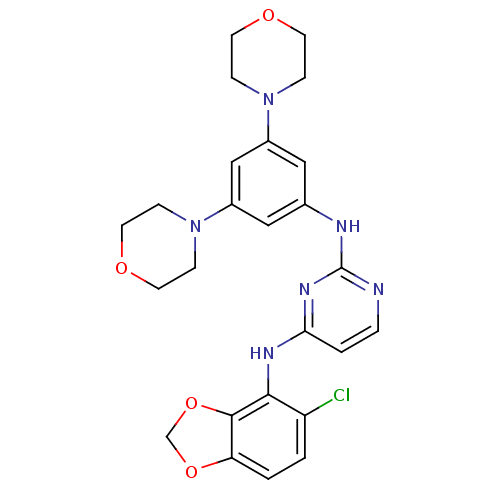
(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data