 Found 51 hits with Last Name = 'morris' and Initial = 'aj'
Found 51 hits with Last Name = 'morris' and Initial = 'aj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501623

(CHEMBL4062929)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@@H]1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)39-26-10-13-40(19-26)34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,43)/t21-,23+,26-,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Competitive inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in LPC hydrolysis measured every 30 secs for 90 mins by Lin... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB2 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human cannabinoid CB1 receptor expressed in insect Sf9 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG channel in HEK293 cells |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 cells using FS-3 as substrate after 15 mins |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501623

(CHEMBL4062929)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@@H]1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)39-26-10-13-40(19-26)34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,43)/t21-,23+,26-,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501636

(CHEMBL4093316)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCC(O)(CNC(=O)OCc2cc(Cl)cc(Cl)c2)CC1 |r| Show InChI InChI=1S/C38H56Cl2N2O6/c1-23(29-5-6-30-34-31(9-11-37(29,30)3)36(2)10-8-28(43)18-25(36)19-32(34)44)4-7-33(45)42-14-12-38(47,13-15-42)22-41-35(46)48-21-24-16-26(39)20-27(40)17-24/h16-17,20,23,25,28-32,34,43-44,47H,4-15,18-19,21-22H2,1-3H3,(H,41,46)/t23-,25+,28-,29-,30+,31+,32+,34+,36+,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501631
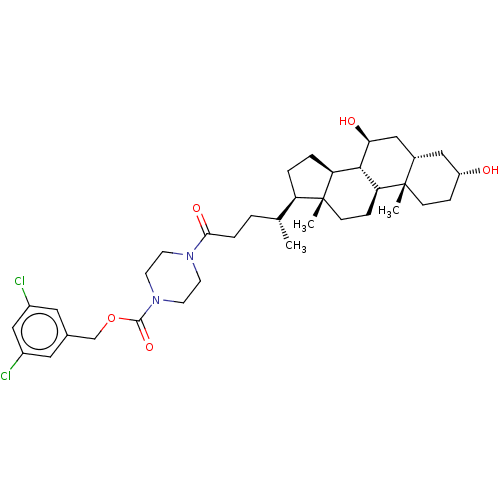
(CHEMBL4081367)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-22(4-7-32(43)39-12-14-40(15-13-39)34(44)45-21-23-16-25(37)20-26(38)17-23)28-5-6-29-33-30(9-11-36(28,29)3)35(2)10-8-27(41)18-24(35)19-31(33)42/h16-17,20,22,24,27-31,33,41-42H,4-15,18-19,21H2,1-3H3/t22-,24+,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501630
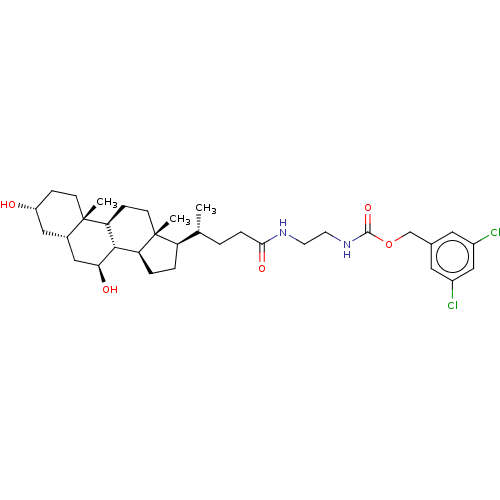
(CHEMBL4105619)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCNC(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C34H50Cl2N2O5/c1-20(4-7-30(41)37-12-13-38-32(42)43-19-21-14-23(35)18-24(36)15-21)26-5-6-27-31-28(9-11-34(26,27)3)33(2)10-8-25(39)16-22(33)17-29(31)40/h14-15,18,20,22,25-29,31,39-40H,4-13,16-17,19H2,1-3H3,(H,37,41)(H,38,42)/t20-,22+,25-,26-,27+,28+,29+,31+,33+,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501624
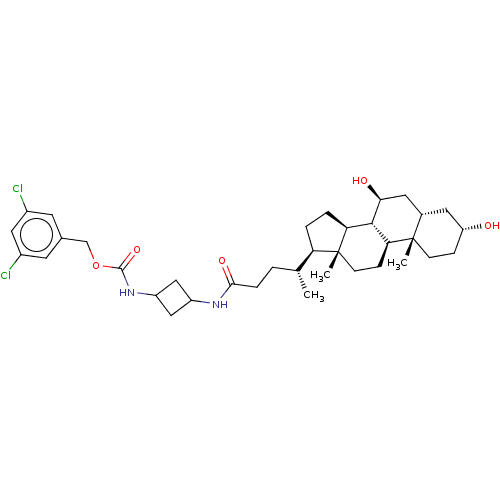
(CHEMBL4090866)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NC1CC(C1)NC(=O)OCc1cc(Cl)cc(Cl)c1 |r,wU:8.8,24.28,18.19,11.11,6.6,wD:14.14,26.30,20.22,4.4,1.0,(53.49,-51.31,;51.96,-51.31,;52.86,-52.55,;51.96,-53.81,;50.5,-53.34,;50.48,-54.87,;49.16,-54.11,;49.15,-52.56,;49.16,-55.65,;50.5,-56.44,;47.82,-56.42,;46.49,-55.66,;46.48,-57.19,;45.16,-56.43,;43.83,-55.65,;42.48,-56.44,;43.83,-54.1,;45.16,-53.33,;46.49,-54.1,;46.48,-52.56,;47.82,-53.33,;47.81,-54.87,;47.82,-51.78,;49.15,-51.01,;50.5,-51.79,;50.49,-50.25,;52.43,-49.84,;51.4,-48.7,;53.93,-49.52,;54.41,-48.05,;55.91,-47.72,;56.94,-48.86,;56.38,-46.26,;57.89,-45.94,;59.17,-46.78,;60.01,-45.49,;58.72,-44.65,;61.52,-45.17,;62.55,-46.31,;62.07,-47.78,;64.06,-45.99,;65.09,-47.13,;66.59,-46.81,;67.06,-45.36,;68.56,-45.03,;69.03,-43.56,;69.6,-46.18,;69.12,-47.64,;70.15,-48.79,;67.62,-47.96,)| Show InChI InChI=1S/C36H52Cl2N2O5/c1-20(4-7-32(43)39-25-17-26(18-25)40-34(44)45-19-21-12-23(37)16-24(38)13-21)28-5-6-29-33-30(9-11-36(28,29)3)35(2)10-8-27(41)14-22(35)15-31(33)42/h12-13,16,20,22,25-31,33,41-42H,4-11,14-15,17-19H2,1-3H3,(H,39,43)(H,40,44)/t20-,22+,25?,26?,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 208 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501633
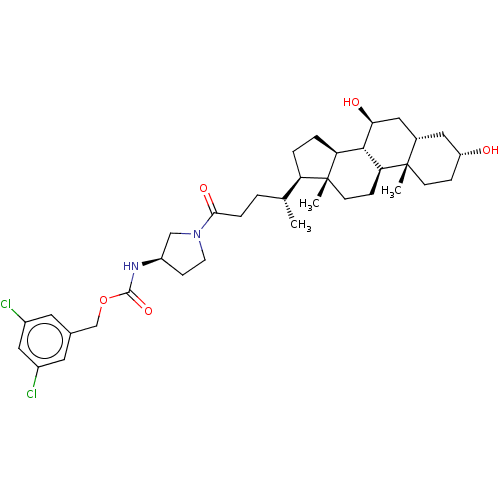
(CHEMBL4071554)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CC[C@H](C1)NC(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)40-13-10-26(19-40)39-34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,44)/t21-,23+,26-,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501632
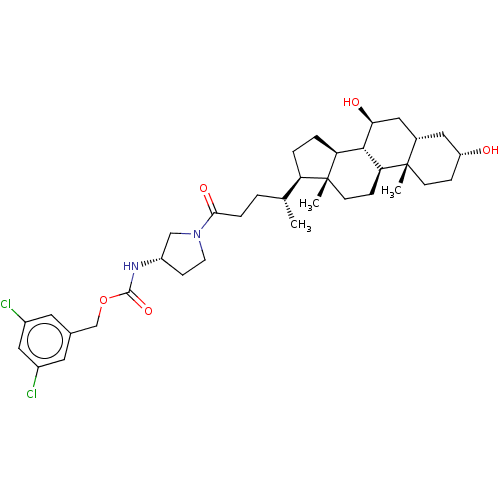
(CHEMBL4094214)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CC[C@@H](C1)NC(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)40-13-10-26(19-40)39-34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,44)/t21-,23+,26+,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501629

(CHEMBL4070768)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCCNC(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C35H52Cl2N2O5/c1-21(5-8-31(42)38-13-4-14-39-33(43)44-20-22-15-24(36)19-25(37)16-22)27-6-7-28-32-29(10-12-35(27,28)3)34(2)11-9-26(40)17-23(34)18-30(32)41/h15-16,19,21,23,26-30,32,40-41H,4-14,17-18,20H2,1-3H3,(H,38,42)(H,39,43)/t21-,23+,26-,27-,28+,29+,30+,32+,34+,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 563 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501634
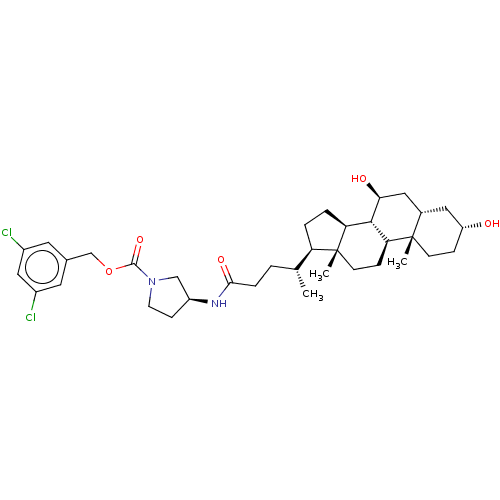
(CHEMBL4098586)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@H]1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H52Cl2N2O5/c1-21(4-7-32(43)39-26-10-13-40(19-26)34(44)45-20-22-14-24(37)18-25(38)15-22)28-5-6-29-33-30(9-12-36(28,29)3)35(2)11-8-27(41)16-23(35)17-31(33)42/h14-15,18,21,23,26-31,33,41-42H,4-13,16-17,19-20H2,1-3H3,(H,39,43)/t21-,23+,26+,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 717 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501627
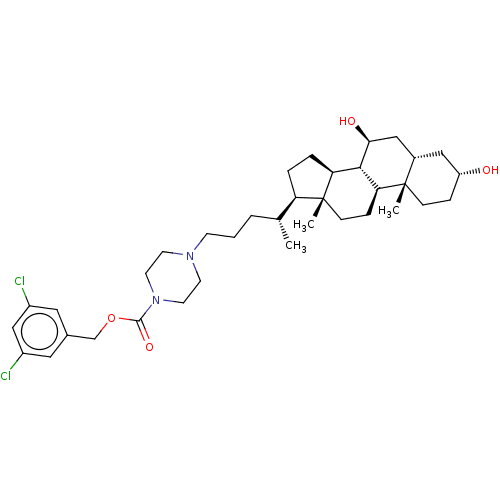
(CHEMBL4085570)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCN1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C36H54Cl2N2O4/c1-23(5-4-12-39-13-15-40(16-14-39)34(43)44-22-24-17-26(37)21-27(38)18-24)29-6-7-30-33-31(9-11-36(29,30)3)35(2)10-8-28(41)19-25(35)20-32(33)42/h17-18,21,23,25,28-33,41-42H,4-16,19-20,22H2,1-3H3/t23-,25+,28-,29-,30+,31+,32+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 814 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501625

(CHEMBL4101049)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NC1CN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C35H50Cl2N2O5/c1-20(4-7-31(42)38-25-17-39(18-25)33(43)44-19-21-12-23(36)16-24(37)13-21)27-5-6-28-32-29(9-11-35(27,28)3)34(2)10-8-26(40)14-22(34)15-30(32)41/h12-13,16,20,22,25-30,32,40-41H,4-11,14-15,17-19H2,1-3H3,(H,38,42)/t20-,22+,26-,27-,28+,29+,30+,32+,34+,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501635
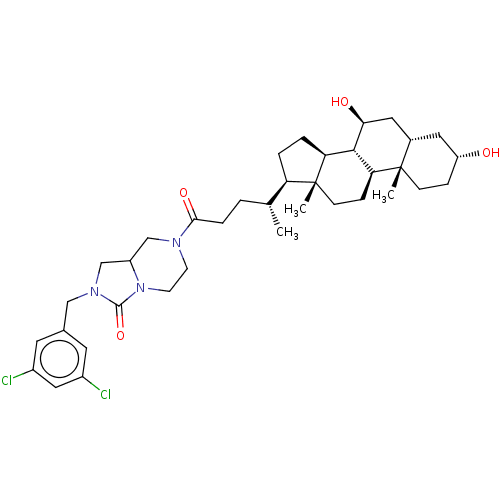
(CHEMBL4083075)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCN2C(CN(Cc3cc(Cl)cc(Cl)c3)C2=O)C1 |r| Show InChI InChI=1S/C37H53Cl2N3O4/c1-22(29-5-6-30-34-31(9-11-37(29,30)3)36(2)10-8-28(43)16-24(36)17-32(34)44)4-7-33(45)40-12-13-42-27(20-40)21-41(35(42)46)19-23-14-25(38)18-26(39)15-23/h14-15,18,22,24,27-32,34,43-44H,4-13,16-17,19-21H2,1-3H3/t22-,24+,27?,28-,29-,30+,31+,32+,34+,36+,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501626
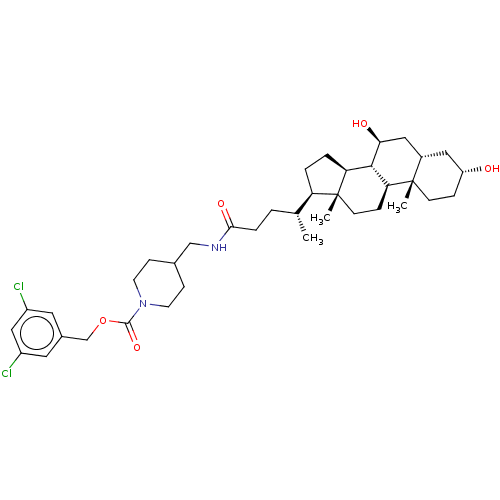
(CHEMBL4072603)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C38H56Cl2N2O5/c1-23(30-5-6-31-35-32(9-13-38(30,31)3)37(2)12-8-29(43)18-26(37)19-33(35)44)4-7-34(45)41-21-24-10-14-42(15-11-24)36(46)47-22-25-16-27(39)20-28(40)17-25/h16-17,20,23-24,26,29-33,35,43-44H,4-15,18-19,21-22H2,1-3H3,(H,41,45)/t23-,26+,29-,30-,31+,32+,33+,35+,37+,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50236230

(2-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dih...)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C26H45NO6S/c1-16(4-7-23(30)27-12-13-34(31,32)33)19-5-6-20-24-21(9-11-26(19,20)3)25(2)10-8-18(28)14-17(25)15-22(24)29/h16-22,24,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33)/t16-,17+,18-,19-,20+,21+,22+,24+,25+,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501622
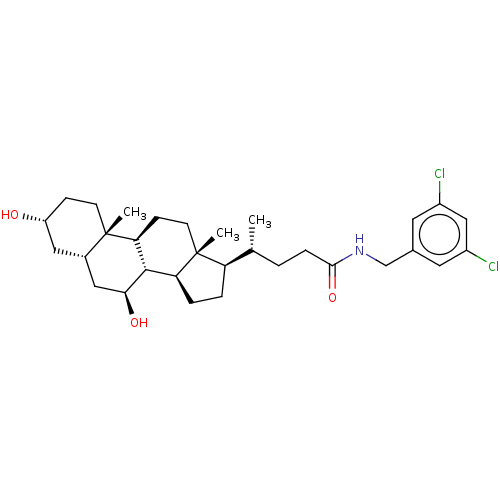
(CHEMBL4097881)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C31H45Cl2NO3/c1-18(4-7-28(37)34-17-19-12-21(32)16-22(33)13-19)24-5-6-25-29-26(9-11-31(24,25)3)30(2)10-8-23(35)14-20(30)15-27(29)36/h12-13,16,18,20,23-27,29,35-36H,4-11,14-15,17H2,1-3H3,(H,34,37)/t18-,20+,23-,24-,25+,26+,27+,29+,30+,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501620
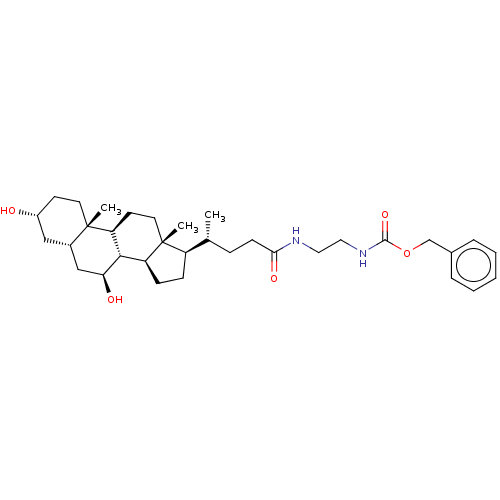
(CHEMBL4078911)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCNC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C34H52N2O5/c1-22(9-12-30(39)35-17-18-36-32(40)41-21-23-7-5-4-6-8-23)26-10-11-27-31-28(14-16-34(26,27)3)33(2)15-13-25(37)19-24(33)20-29(31)38/h4-8,22,24-29,31,37-38H,9-21H2,1-3H3,(H,35,39)(H,36,40)/t22-,24+,25-,26-,27+,28+,29+,31+,33+,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501628

(CHEMBL4064007)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N1CCN(CC1)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C36H54N2O5/c1-24(9-12-32(41)37-17-19-38(20-18-37)34(42)43-23-25-7-5-4-6-8-25)28-10-11-29-33-30(14-16-36(28,29)3)35(2)15-13-27(39)21-26(35)22-31(33)40/h4-8,24,26-31,33,39-40H,9-23H2,1-3H3/t24-,26+,27-,28-,29+,30+,31+,33+,35+,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50501621
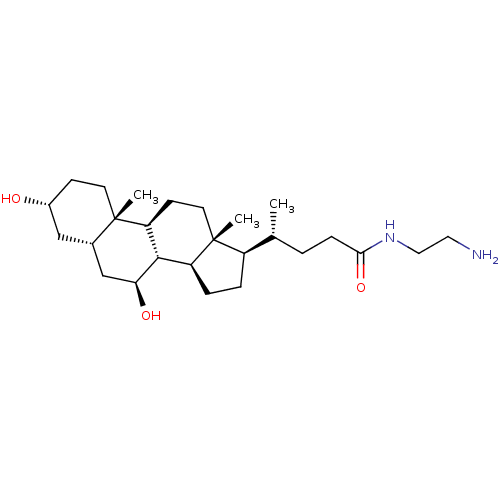
(CHEMBL4096938)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCN |r| Show InChI InChI=1S/C26H46N2O3/c1-16(4-7-23(31)28-13-12-27)19-5-6-20-24-21(9-11-26(19,20)3)25(2)10-8-18(29)14-17(25)15-22(24)30/h16-22,24,29-30H,4-15,27H2,1-3H3,(H,28,31)/t16-,17+,18-,19-,20+,21+,22+,24+,25+,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Netherlands Institute
Curated by ChEMBL
| Assay Description
Inhibition of human ATX expressed in HEK293 Flp-In cells assessed as decrease in choline release from LPC measured every 30 secs for 90 mins by HVA b... |
J Med Chem 60: 2006-2017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01743
BindingDB Entry DOI: 10.7270/Q22F7RGR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418023
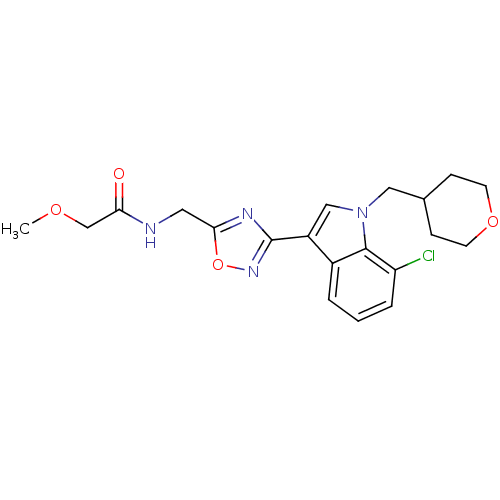
(CHEMBL1682276)Show SMILES COCC(=O)NCc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H23ClN4O4/c1-27-12-17(26)22-9-18-23-20(24-29-18)15-11-25(10-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,11,13H,5-10,12H2,1H3,(H,22,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418022

(CHEMBL1682270)Show SMILES COCCN(C)Cc1nc(ns1)-c1cn(CC2CCS(=O)(=O)CC2)c2c(Cl)cccc12 Show InChI InChI=1S/C21H27ClN4O3S2/c1-25(8-9-29-2)14-19-23-21(24-30-19)17-13-26(20-16(17)4-3-5-18(20)22)12-15-6-10-31(27,28)11-7-15/h3-5,13,15H,6-12,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418021
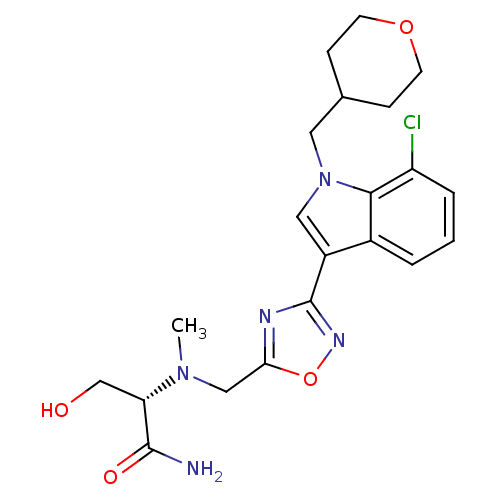
(CHEMBL1682274)Show SMILES CN(Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12)[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C21H26ClN5O4/c1-26(17(12-28)20(23)29)11-18-24-21(25-31-18)15-10-27(9-13-5-7-30-8-6-13)19-14(15)3-2-4-16(19)22/h2-4,10,13,17,28H,5-9,11-12H2,1H3,(H2,23,29)/t17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418020

(CHEMBL1682266)Show SMILES NC(=O)CNC(=O)[C@@H]1CCCN1Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 |r| Show InChI InChI=1S/C24H29ClN6O3S/c25-18-4-1-3-16-17(13-31(22(16)18)12-15-6-9-34-10-7-15)23-28-21(35-29-23)14-30-8-2-5-19(30)24(33)27-11-20(26)32/h1,3-4,13,15,19H,2,5-12,14H2,(H2,26,32)(H,27,33)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418019
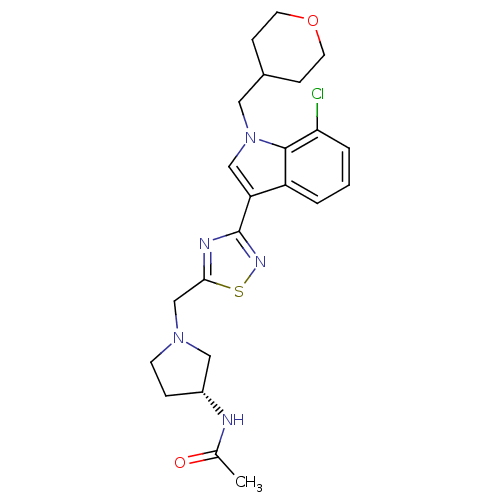
(CHEMBL1682263)Show SMILES CC(=O)N[C@@H]1CCN(Cc2nc(ns2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)C1 |r| Show InChI InChI=1S/C23H28ClN5O2S/c1-15(30)25-17-5-8-28(12-17)14-21-26-23(27-32-21)19-13-29(11-16-6-9-31-10-7-16)22-18(19)3-2-4-20(22)24/h2-4,13,16-17H,5-12,14H2,1H3,(H,25,30)/t17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418018
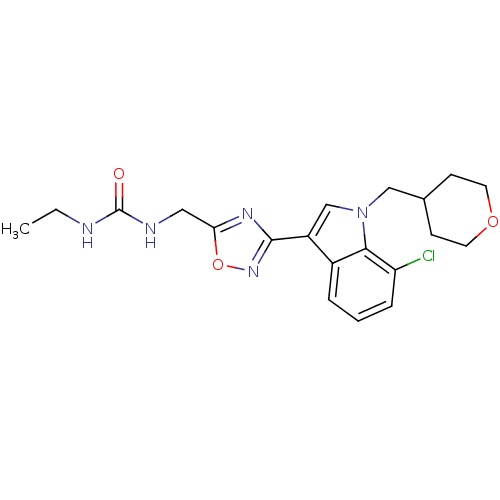
(CHEMBL1682277)Show SMILES CCNC(=O)NCc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-2-22-20(27)23-10-17-24-19(25-29-17)15-12-26(11-13-6-8-28-9-7-13)18-14(15)4-3-5-16(18)21/h3-5,12-13H,2,6-11H2,1H3,(H2,22,23,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418017
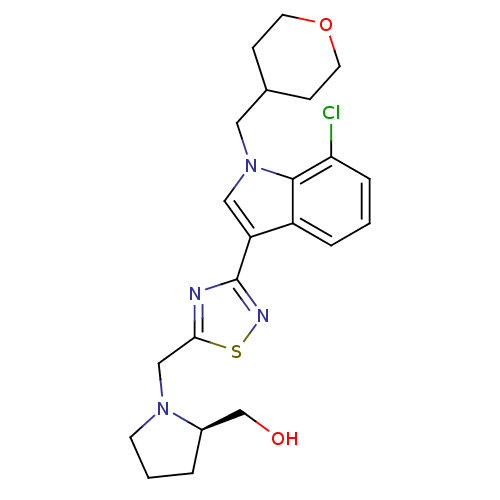
(CHEMBL1682264)Show SMILES OC[C@H]1CCCN1Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 |r| Show InChI InChI=1S/C22H27ClN4O2S/c23-19-5-1-4-17-18(12-27(21(17)19)11-15-6-9-29-10-7-15)22-24-20(30-25-22)13-26-8-2-3-16(26)14-28/h1,4-5,12,15-16,28H,2-3,6-11,13-14H2/t16-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418016
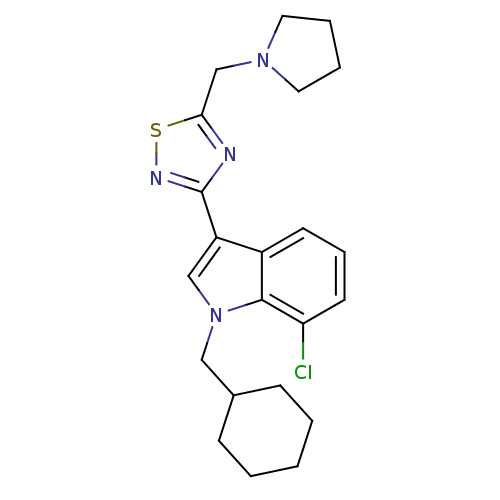
(CHEMBL1682261)Show SMILES Clc1cccc2c(cn(CC3CCCCC3)c12)-c1nsc(CN2CCCC2)n1 Show InChI InChI=1S/C22H27ClN4S/c23-19-10-6-9-17-18(14-27(21(17)19)13-16-7-2-1-3-8-16)22-24-20(28-25-22)15-26-11-4-5-12-26/h6,9-10,14,16H,1-5,7-8,11-13,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418015
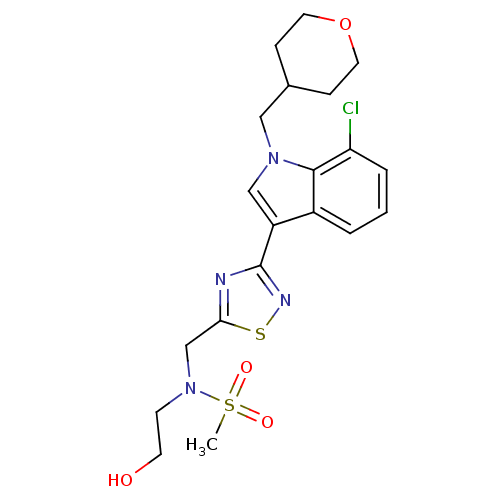
(CHEMBL1682269)Show SMILES CS(=O)(=O)N(CCO)Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H25ClN4O4S2/c1-31(27,28)25(7-8-26)13-18-22-20(23-30-18)16-12-24(11-14-5-9-29-10-6-14)19-15(16)3-2-4-17(19)21/h2-4,12,14,26H,5-11,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418014
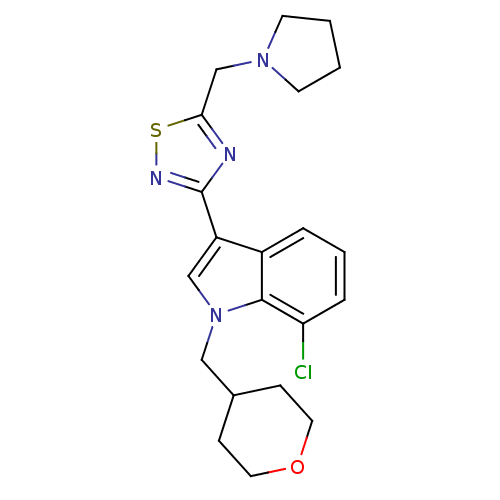
(CHEMBL1682262)Show SMILES Clc1cccc2c(cn(CC3CCOCC3)c12)-c1nsc(CN2CCCC2)n1 Show InChI InChI=1S/C21H25ClN4OS/c22-18-5-3-4-16-17(13-26(20(16)18)12-15-6-10-27-11-7-15)21-23-19(28-24-21)14-25-8-1-2-9-25/h3-5,13,15H,1-2,6-12,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50416472

(CHEMBL1209708)Show SMILES COc1cccc2c(cn(CC3CCCCC3)c12)C(=O)N1C[C@H](C)N(C)[C@H](C)C1 |r| Show InChI InChI=1S/C24H35N3O2/c1-17-13-27(14-18(2)25(17)3)24(28)21-16-26(15-19-9-6-5-7-10-19)23-20(21)11-8-12-22(23)29-4/h8,11-12,16-19H,5-7,9-10,13-15H2,1-4H3/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418013

(CHEMBL1682278)Show SMILES CN(C)S(=O)(=O)N(C)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H26ClN5O4S/c1-24(2)31(27,28)25(3)13-18-22-20(23-30-18)16-12-26(11-14-7-9-29-10-8-14)19-15(16)5-4-6-17(19)21/h4-6,12,14H,7-11,13H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418012

(CHEMBL1682272)Show SMILES CN(CC(N)=O)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H24ClN5O3/c1-25(11-17(22)27)12-18-23-20(24-29-18)15-10-26(9-13-5-7-28-8-6-13)19-14(15)3-2-4-16(19)21/h2-4,10,13H,5-9,11-12H2,1H3,(H2,22,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418011

(CHEMBL1682273)Show SMILES CN(CC(=O)NCCO)Cc1nc(no1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C22H28ClN5O4/c1-27(13-19(30)24-7-8-29)14-20-25-22(26-32-20)17-12-28(11-15-5-9-31-10-6-15)21-16(17)3-2-4-18(21)23/h2-4,12,15,29H,5-11,13-14H2,1H3,(H,24,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418010
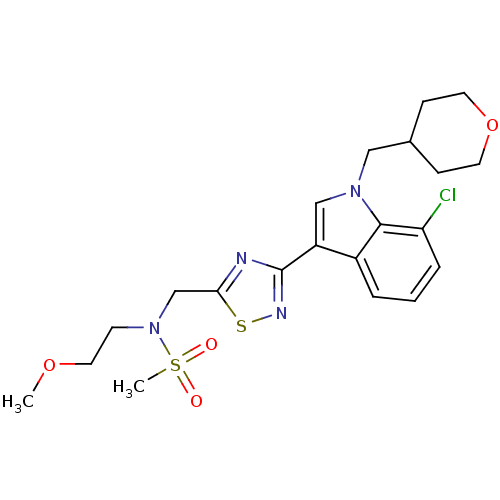
(CHEMBL1682268)Show SMILES COCCN(Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12)S(C)(=O)=O Show InChI InChI=1S/C21H27ClN4O4S2/c1-29-11-8-26(32(2,27)28)14-19-23-21(24-31-19)17-13-25(12-15-6-9-30-10-7-15)20-16(17)4-3-5-18(20)22/h3-5,13,15H,6-12,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418009
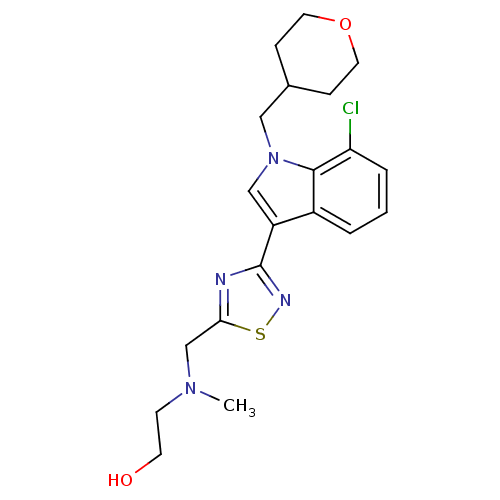
(CHEMBL1682267)Show SMILES CN(CCO)Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C20H25ClN4O2S/c1-24(7-8-26)13-18-22-20(23-28-18)16-12-25(11-14-5-9-27-10-6-14)19-15(16)3-2-4-17(19)21/h2-4,12,14,26H,5-11,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418008

(CHEMBL1681794)Show SMILES COCCN(C)Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C21H27ClN4O2S/c1-25(8-11-27-2)14-19-23-21(24-29-19)17-13-26(12-15-6-9-28-10-7-15)20-16(17)4-3-5-18(20)22/h3-5,13,15H,6-12,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418007
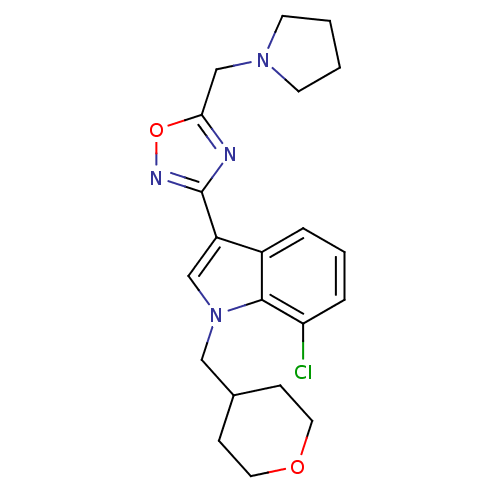
(CHEMBL1682271)Show SMILES Clc1cccc2c(cn(CC3CCOCC3)c12)-c1noc(CN2CCCC2)n1 Show InChI InChI=1S/C21H25ClN4O2/c22-18-5-3-4-16-17(13-26(20(16)18)12-15-6-10-27-11-7-15)21-23-19(28-24-21)14-25-8-1-2-9-25/h3-5,13,15H,1-2,6-12,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50418006
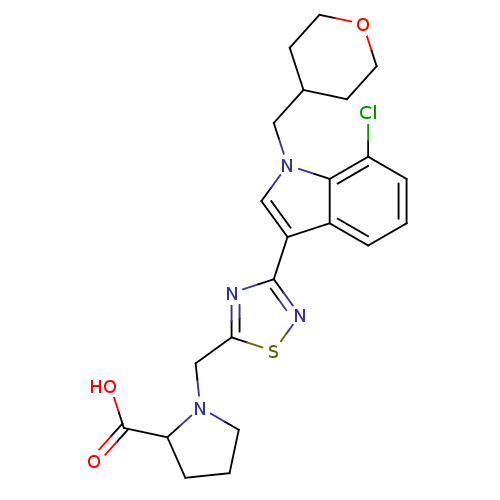
(CHEMBL1682265)Show SMILES OC(=O)C1CCCN1Cc1nc(ns1)-c1cn(CC2CCOCC2)c2c(Cl)cccc12 Show InChI InChI=1S/C22H25ClN4O3S/c23-17-4-1-3-15-16(12-27(20(15)17)11-14-6-9-30-10-7-14)21-24-19(31-25-21)13-26-8-2-5-18(26)22(28)29/h1,3-4,12,14,18H,2,5-11,13H2,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 1748-53 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.082
BindingDB Entry DOI: 10.7270/Q2HD7WWG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data