

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50449636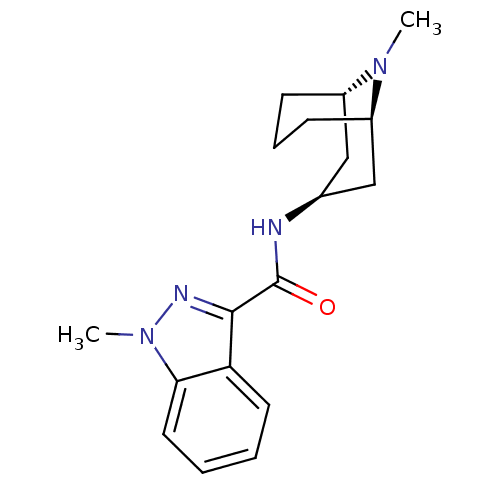 (BRL-43694 | GRANISETRON | Kytril | LY-278584 | San...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014407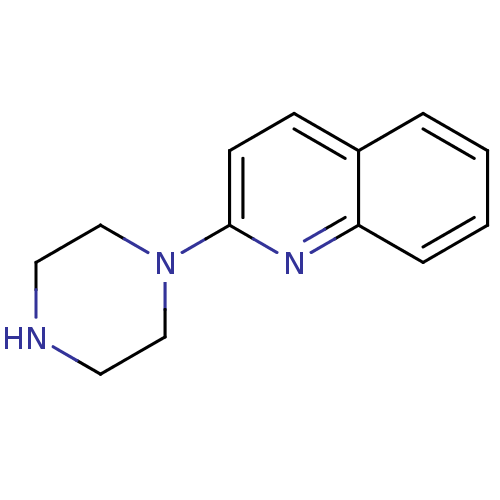 (2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392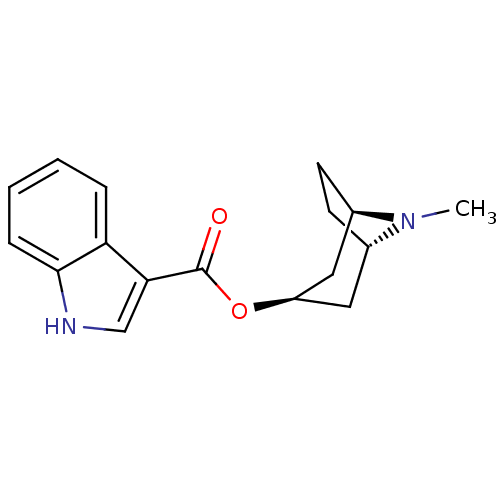 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM85330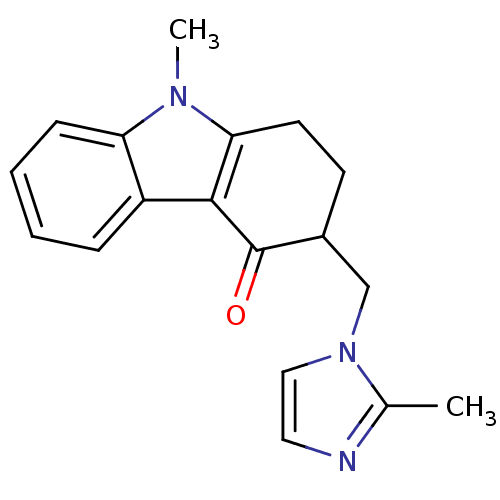 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM82561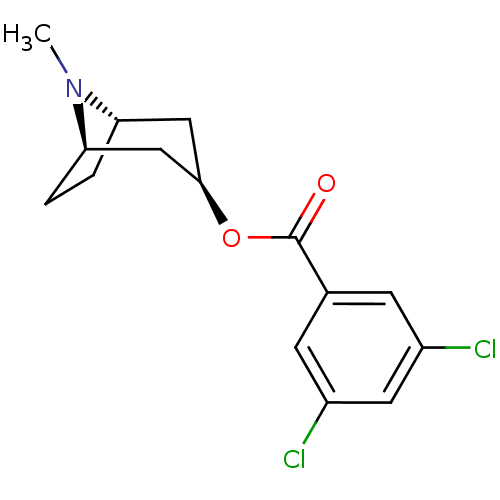 (CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM48320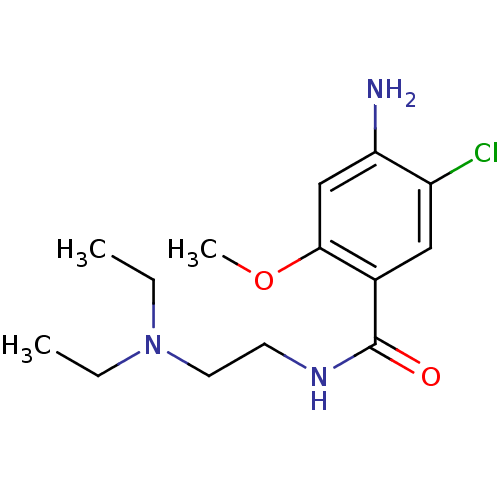 (4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062449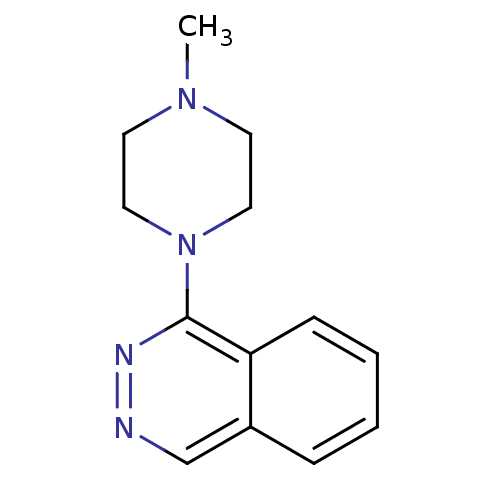 (1-(4-Methyl-piperazin-1-yl)-phthalazine; hydrochlo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M1 by measuring displacement of [3H]- pirenzepine from rat hipp... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062450 (1-(4-Methyl-piperazin-1-yl)-4-phenyl-phthalazine; ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M1 by measuring displacement of [3H]- pirenzepine from rat hipp... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062447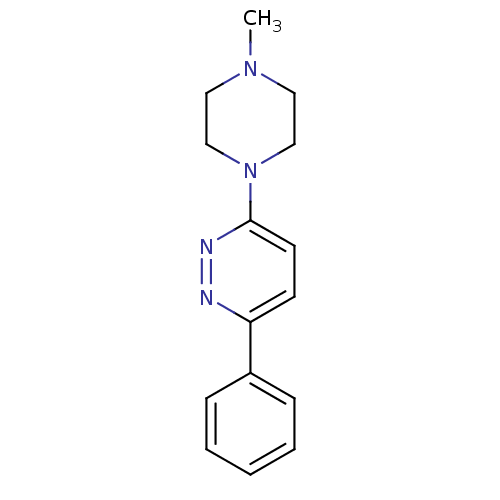 (3-(4-Methyl-piperazin-1-yl)-6-phenyl-pyridazine; h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M1 by measuring displacement of [3H]- pirenzepine from rat hipp... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50062449 (1-(4-Methyl-piperazin-1-yl)-phthalazine; hydrochlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M2 by measuring displacement of [3H]- NMS ligand from rat cardi... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50062451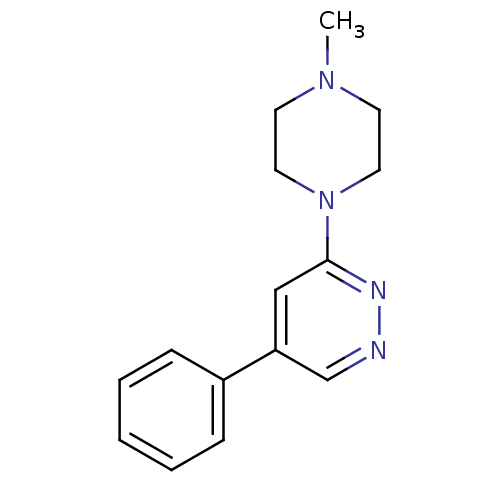 (3-(4-Methyl-piperazin-1-yl)-5-phenyl-pyridazine; h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M1 by measuring displacement of [3H]- pirenzepine from rat hipp... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50062447 (3-(4-Methyl-piperazin-1-yl)-6-phenyl-pyridazine; h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M2 by measuring displacement of [3H]- NMS ligand from rat cardi... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50062451 (3-(4-Methyl-piperazin-1-yl)-5-phenyl-pyridazine; h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M2 by measuring displacement of [3H]- NMS ligand from rat cardi... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50062450 (1-(4-Methyl-piperazin-1-yl)-4-phenyl-phthalazine; ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for Muscarinic acetylcholine receptor M2 by measuring displacement of [3H]- NMS ligand from rat cardi... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50062446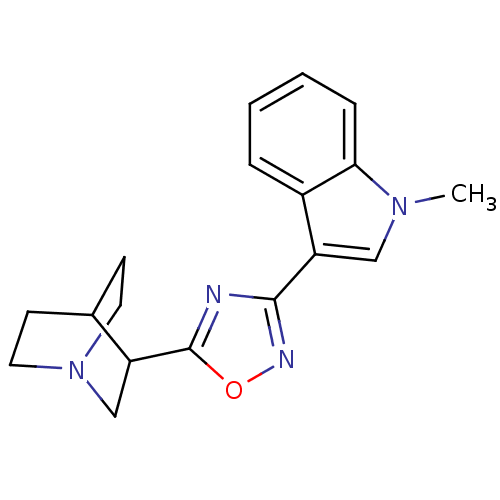 (3-[3-(1-Methyl-1H-indol-3-yl)-[1,2,4]oxadiazol-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement [3H]GR-65630 in rat cerebral cortex. | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50062449 (1-(4-Methyl-piperazin-1-yl)-phthalazine; hydrochlo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement of [3H]granisetron from rat cerebral cor... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50062451 (3-(4-Methyl-piperazin-1-yl)-5-phenyl-pyridazine; h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement of [3H]granisetron from rat cerebral cor... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50062450 (1-(4-Methyl-piperazin-1-yl)-4-phenyl-phthalazine; ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement of [3H]granisetron from rat cerebral cor... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50062447 (3-(4-Methyl-piperazin-1-yl)-6-phenyl-pyridazine; h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity for 5-hydroxytryptamine 3 receptor by measuring displacement of [3H]granisetron from rat cerebral cor... | J Med Chem 41: 311-7 (1998) Article DOI: 10.1021/jm9705418 BindingDB Entry DOI: 10.7270/Q2N29XMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM86004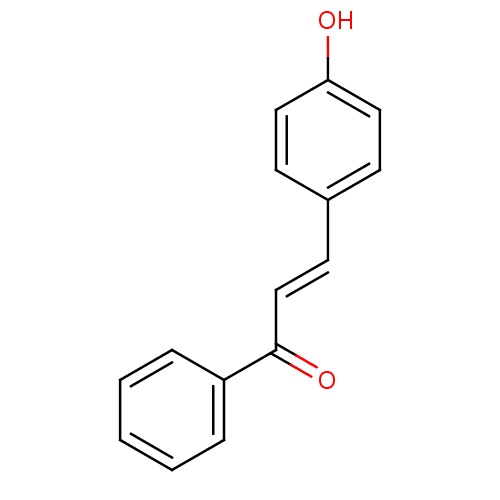 (Chalcone 2 | Chalcone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | 20 |
Université Louis Pasteur | Assay Description Binding of chalcone 4 and chlcone 1 to CSCL12 was examined by monitoring changes in the emission intensity of intrinsic Trp fluorescence of the chemo... | J Biol Chem 283: 23189-99 (2008) Article DOI: 10.1074/jbc.M803947200 BindingDB Entry DOI: 10.7270/Q23X858J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50042978 ((E)-1-(2-hydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | 20 |
Université Louis Pasteur | Assay Description Binding of chalcone 4 and chlcone 1 to CSCL12 was examined by monitoring changes in the emission intensity of intrinsic Trp fluorescence of the chemo... | J Biol Chem 283: 23189-99 (2008) Article DOI: 10.1074/jbc.M803947200 BindingDB Entry DOI: 10.7270/Q23X858J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50042947 (1-(4-Chloro-phenyl)-3-(4-hydroxy-3-methoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | 20 |
Université Louis Pasteur | Assay Description Binding of chalcone 4 and chlcone 1 to CSCL12 was examined by monitoring changes in the emission intensity of intrinsic Trp fluorescence of the chemo... | J Biol Chem 283: 23189-99 (2008) Article DOI: 10.1074/jbc.M803947200 BindingDB Entry DOI: 10.7270/Q23X858J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM29143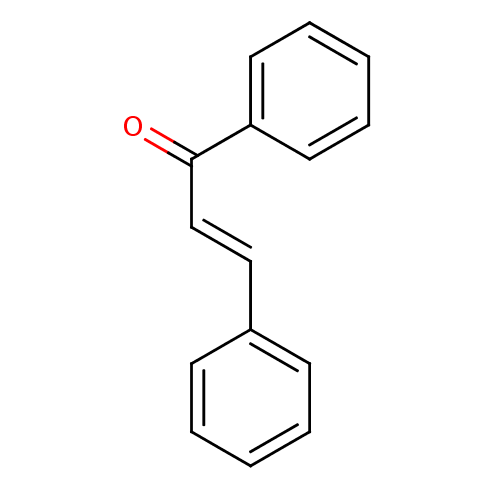 (CHEMBL7976 | Chalcone 1 | Chalcone, 13 | cid_63776...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | 20 |
Université Louis Pasteur | Assay Description Binding of chalcone 4 and chlcone 1 to CSCL12 was examined by monitoring changes in the emission intensity of intrinsic Trp fluorescence of the chemo... | J Biol Chem 283: 23189-99 (2008) Article DOI: 10.1074/jbc.M803947200 BindingDB Entry DOI: 10.7270/Q23X858J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM150752 (4-hydrazino-5,6,7,8-tetrahydro[1]benzothieno[2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.83E+4 | n/a | n/a | n/a | 7.5 | 30 |
Université Pierre et Marie Curie | Assay Description We incubated human recombinant CDK2/cyclin A with small molecules at the indicatedconcentrations in buffer A (Tris¿¿HCl (25 mm, pH 7.5), MgCl2 (10 mm... | Chembiochem 16: 432-9 (2015) Article DOI: 10.1002/cbic.201402579 BindingDB Entry DOI: 10.7270/Q2T43RTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM150753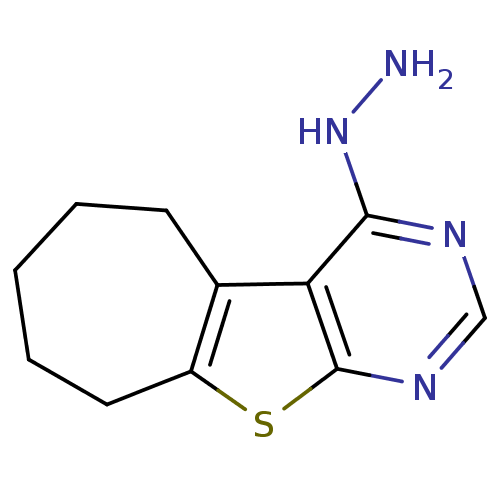 (4-hydrazino-6,7,8,9-tetrahydro-5H-cyclohepta[4,5]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | 7.5 | 30 |
Université Pierre et Marie Curie | Assay Description We incubated human recombinant CDK2/cyclin A with small molecules at the indicatedconcentrations in buffer A (Tris¿¿HCl (25 mm, pH 7.5), MgCl2 (10 mm... | Chembiochem 16: 432-9 (2015) Article DOI: 10.1002/cbic.201402579 BindingDB Entry DOI: 10.7270/Q2T43RTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM43654 (7,8-dihydro-6H-cyclopenta[4,5]thieno[1,2-c]pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.87E+5 | n/a | n/a | n/a | 7.5 | 30 |
Université Pierre et Marie Curie | Assay Description We incubated human recombinant CDK2/cyclin A with small molecules at the indicatedconcentrations in buffer A (Tris¿¿HCl (25 mm, pH 7.5), MgCl2 (10 mm... | Chembiochem 16: 432-9 (2015) Article DOI: 10.1002/cbic.201402579 BindingDB Entry DOI: 10.7270/Q2T43RTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM32021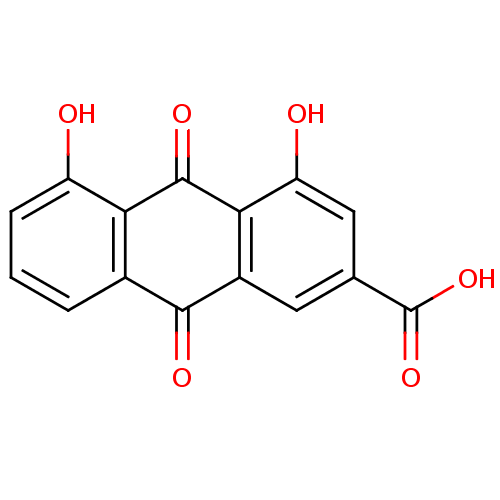 (4,5-bis(oxidanyl)-9,10-bis(oxidanylidene)anthracen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | 7.5 | 30 |
Université Pierre et Marie Curie | Assay Description We incubated human recombinant CDK2/cyclin A with small molecules at the indicatedconcentrations in buffer A (Tris¿¿HCl (25 mm, pH 7.5), MgCl2 (10 mm... | Chembiochem 16: 432-9 (2015) Article DOI: 10.1002/cbic.201402579 BindingDB Entry DOI: 10.7270/Q2T43RTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM150754 (11-O-acetyl-aloe-emodin | 3-[(acetyloxy)methyl]-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | 7.5 | 30 |
Université Pierre et Marie Curie | Assay Description We incubated human recombinant CDK2/cyclin A with small molecules at the indicatedconcentrations in buffer A (Tris¿¿HCl (25 mm, pH 7.5), MgCl2 (10 mm... | Chembiochem 16: 432-9 (2015) Article DOI: 10.1002/cbic.201402579 BindingDB Entry DOI: 10.7270/Q2T43RTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||